Far-red fluorescent protein and fusion protein thereof
A technology of red light fluorescence and fluorescent protein, which is applied in the field of fluorescent markers, can solve the problems of ineffective combination use and single emission wavelength, and achieves the effect of stable performance and improved effective brightness.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
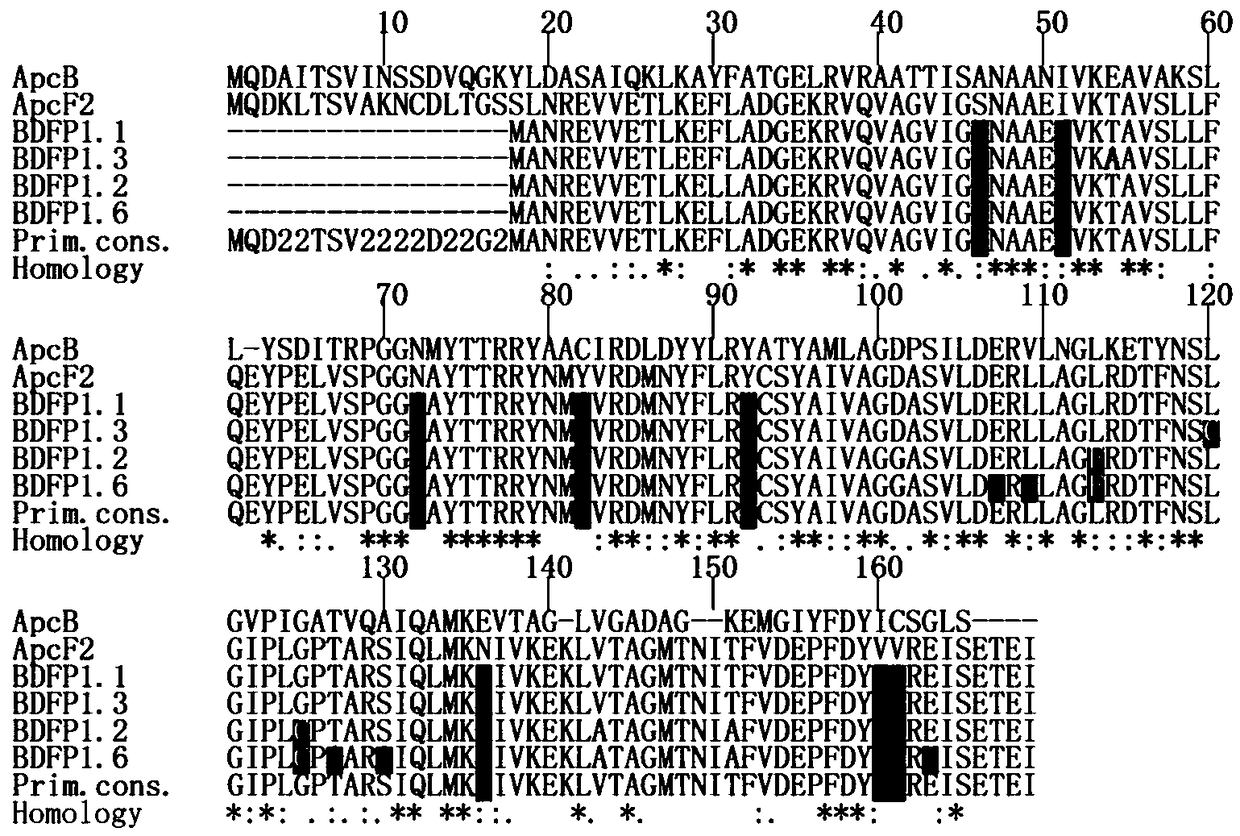
[0058] Example 1. Homologous sequence comparison analysis
[0059] figure 1 A comparison of several homologous sequences is shown, in which ApcF2 and its derivative sequence BDFP1.1 are all from Chroococcidiopsis thermalis PCC 7203; ApcB is from Spirulina platensis, which is not suitable for the growth environment of far-red light and near-infrared light. From figure 1 It can be seen that L113 is highly conserved among ApcB, ApcF2 and BDFP1.1.
[0060] On the basis of the BDFP1.1 sequence (SEQ ID NO: 2), L120 was subjected to site-directed saturation mutagenesis; the screened mutants were subjected to continuous random mutation through an error-prone PCR reaction to establish a mutant library. The mutants in the mutant library were screened by using the method of in vitro spectral property detection and live cell imaging according to the following screening criteria: Compared with BDFP1. 660nm; simultaneously with known fluorescent protein marker iRFP670 (ε·Φ fl =10.2mM -...
Embodiment 2
[0067] Example 2. Detection of the effective brightness of each fluorescent protein in live HEK 293T cells
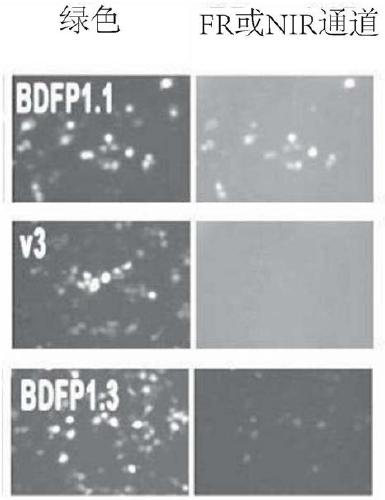
[0068] The nucleic acid (FPs:IRES:eGFP) expressing the mutant BDFP1.1, v3 or v12 (BDFP1.3) was constructed in the expression vector pcDNA3.1, then transiently transfected into HEK 293T cells, and observed using an inverted fluorescence microscope Fluorescence brightness. The results are shown in figure 2 middle.
[0069] figure 2 Fluorescence images observed under the green channel and FR or NIR channel, respectively, are shown. It can be seen that BDFP1.1, v3 or v12 (BDFP1.3) all emit green fluorescence, and BDFP1.1 and v12 (BDFP1.3) emit red fluorescence. Compared with them, the red fluorescence of v3 is weaker.
Embodiment 3
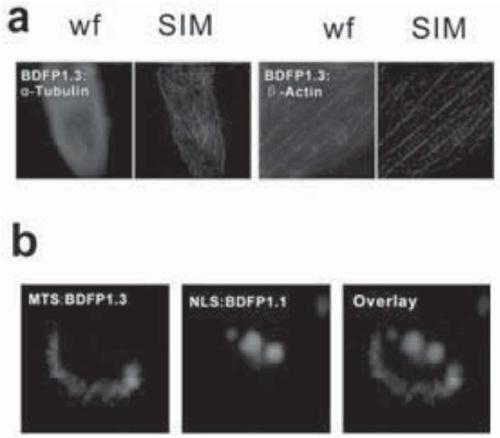
[0070] Embodiment 3. Comparison of the spectral properties of mutant v12 (BDFP1.3) and known fluorescent proteins and the effective brightness in HEK 293T cells
[0071] Fluorescent proteins expressed in E. coli were purified by Ni2+ affinity chromatography, and then their spectral properties were examined. The detection solution environment is KPB (20mM, pH 7.0) and 0.5M NaCl; the excitation light wavelength of the fluorescence emission spectrum is 660nm. The comparison reference substance of molecular brightness is iRFP670(ε·dp fl =10.2mM -1 cm -1 ). In addition, the effective fluorescence (far-red light) of each mutant in HEK293T cells was compared. The results are shown in Table 3 below.
[0072] It can be seen from Table 3 that the emission wavelength of BDFP1.1 is 707nm, while the emission wavelength of BDFP1.3 is 675nm. Therefore, BDFP1.3 can be used in combination with BDFP1.1 for dual fluorescent labeling.
[0073] In addition, BDFP1.3 has an effective brightne...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap