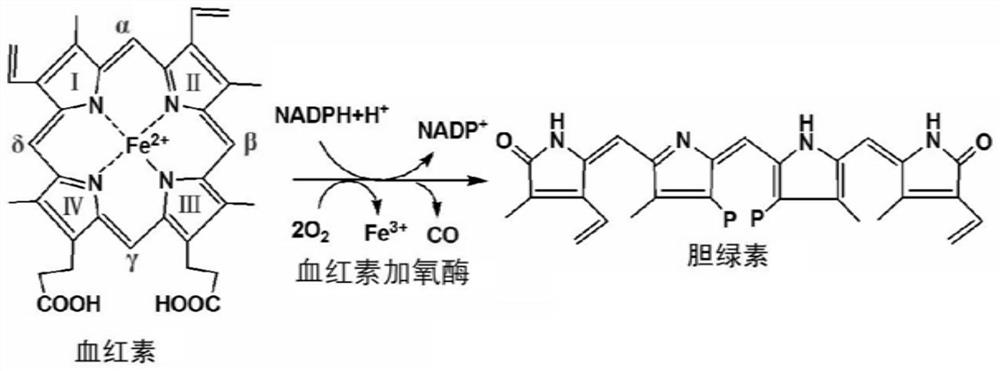
Recombinant Escherichia coli zjut-ho1 and its application in the preparation of biliverdin
A technology for recombining Escherichia coli and biliverdin, applied in bacteria, enzymes, microorganism-based methods, etc., can solve the problems of high price, low yield, difficult industrial application, etc., and achieve short culture period, high activity, and yield. improved effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
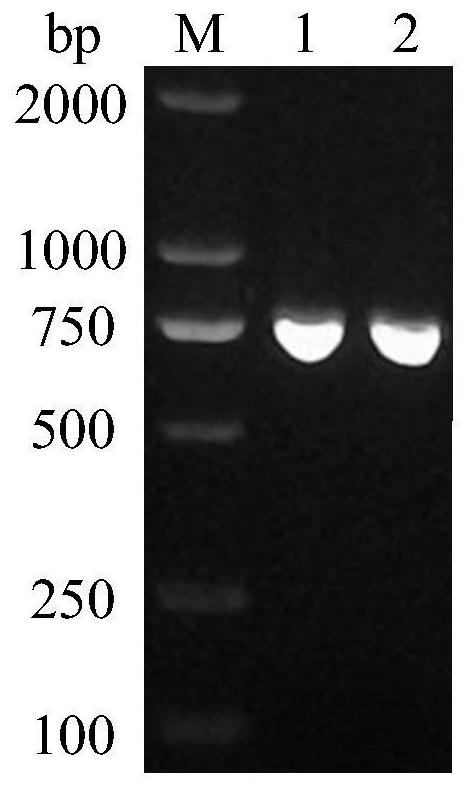
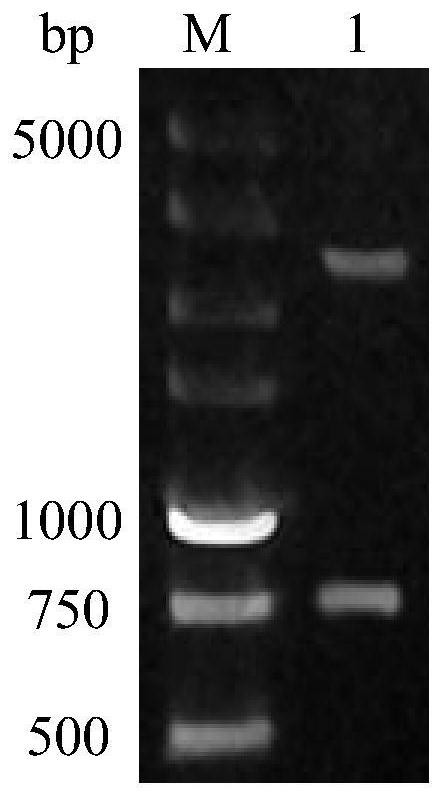
[0035] Example 1 Construction of Gene Recombinant Escherichia coli Expressing HO-1
[0036] The recombinant Escherichia coli zjut-ho1 strain expressing HO-1 of the present invention is constructed according to the following steps:
[0037] (1) After extracting the total RNA of Synechocystis PCC 6803 with "Trizol Univer Total RNA Extraction Reagent", use the "AMV First-Strand cDNA Synthesis Kit" to synthesize the first-strand cDNA by RT-PCR.
[0038] (2) Using the cDNA obtained in step (1) as a template, design primers P1 and P2, introduce restriction endonucleases NdeI and XhoI restriction sites, and amplify the gene (ho1) sequence of HO-1 by PCR. "SanPrep Column PCR Product Purification Kit" is used for purification to obtain the sequence of the target gene ho1. The nucleotide sequence of the gene ho1 is shown in SEQ ID NO:1.
[0039] The primers P1 and P2 are respectively:
[0040] P1: 5′-GGGAATTCCATATGAGTGTCAACTTAGC-3′
[0041] P2: 5'-CCGCTCGAGCTAGCCTTCGGAG-3'.
[0042]...
Embodiment 2
[0059] Embodiment 2: the preferred condition of recombinant Escherichia coli zjut-ho1 strain expressing HO-1
[0060] On the basis of obtaining the Escherichia coli zjut-ho1 strain capable of expressing HO-1 in Example 1, the culture conditions for expressing HO-1 of the strain were optimized, including the type of medium, the time of adding the inducer, and the time of induction culture , the concentration of the inducer added, and the temperature of the induced culture. After optimization, the expression level of HO-1 is significantly improved. The preferred zjut-ho1 strain expresses HO-1 and the process steps are as follows:
[0061] (1) The zjut-ho1 strains that were freeze-dried or glycerol-frozen preserved were inoculated in LB slant medium containing 50 μg / mL kanamycin, and cultured at a constant temperature of 37° C. for 24 hours to obtain slant bacteria; the LB slant culture The final base concentration is composed of: yeast extract powder 5g / L, peptone 10g / L, NaCl 1...
Embodiment 3
[0065] Example 3 Escherichia coli zjut-ho1 is applied to biotransformation of heme to prepare biliverdin
[0066] On the basis of Example 2, the zjut-ho1 bacterium expressing HO-1 was cultivated for the bioconversion of heme to prepare biliverdin. The specific steps are as follows:
[0067] (1) According to the method of embodiment 2, the zjut-ho1 culture fluid (OD) cultivated through HO-1 expression 600 =1.40~1.65), add 2g / L hemin aqueous solution to make the final concentration of hemin 200mg / L, and continue to cultivate at 37°C, 180r / min constant temperature shaking shaker for 5h. The preparation method of the hemin aqueous solution is: 100mg of hemin is slightly heated and dissolved in 50mL with a mass concentration of 0.25% Na 2 CO 3 Aqueous solution, that is, 2g / L hemin aqueous solution.
[0068] (2) The reaction system in step (1) was centrifuged at 1000 g for 10 min to collect the thalline, and the thalline was dark green at this time. The bacteria were suspended i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com