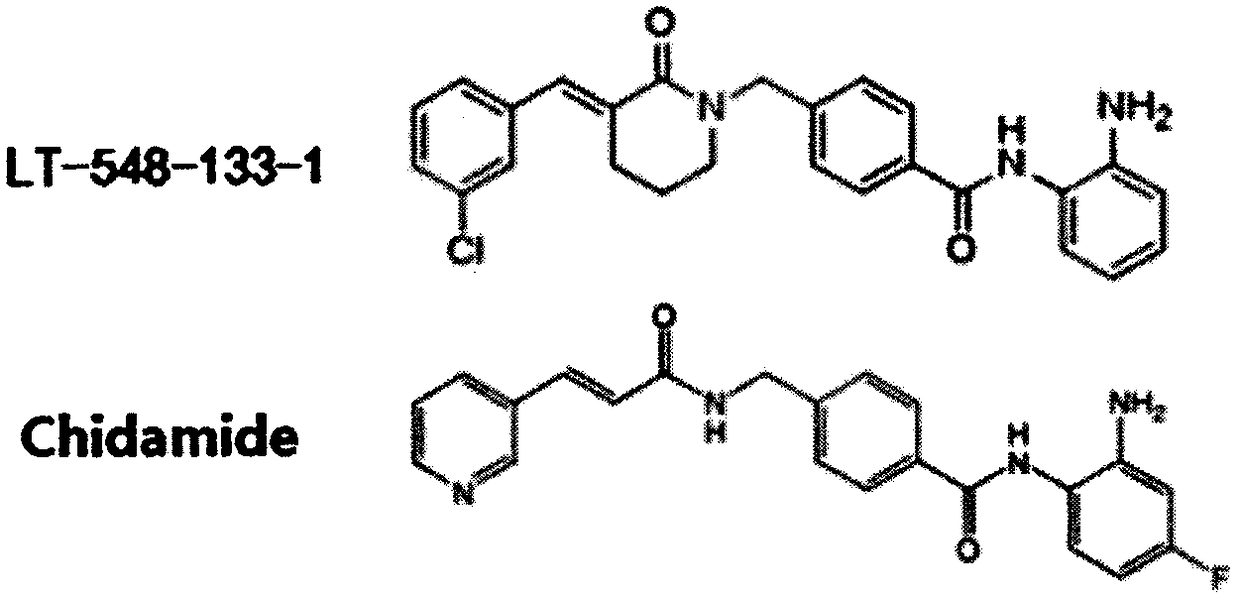
Use of chidamide analogue in preparation of anti-tumor drug
A technology similar in structure to chidamide, applied in the field of biomedicine, can solve problems such as decreased activity of tumor suppressor factors, abnormal gene expression, and abnormal local conformation of chromosomes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
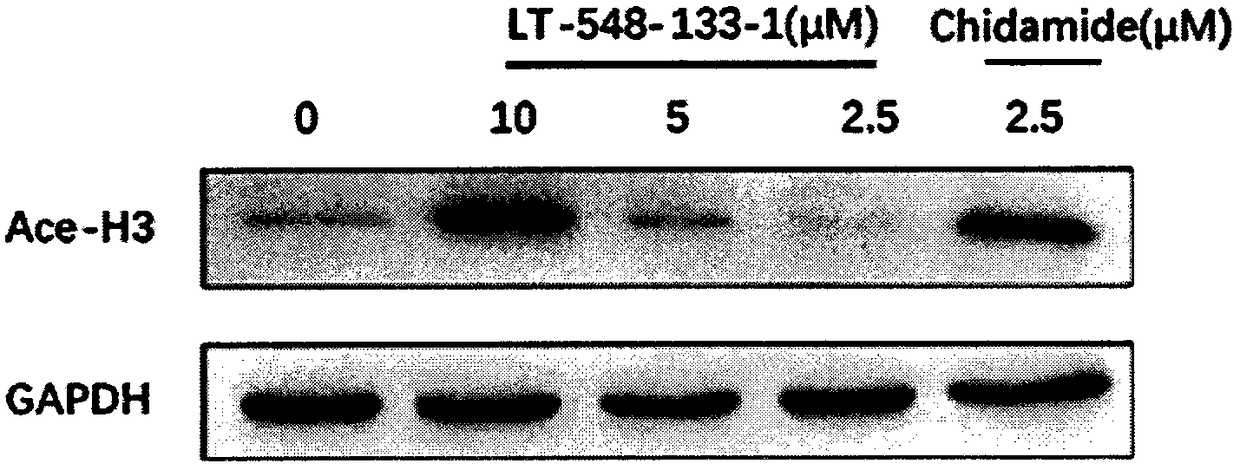
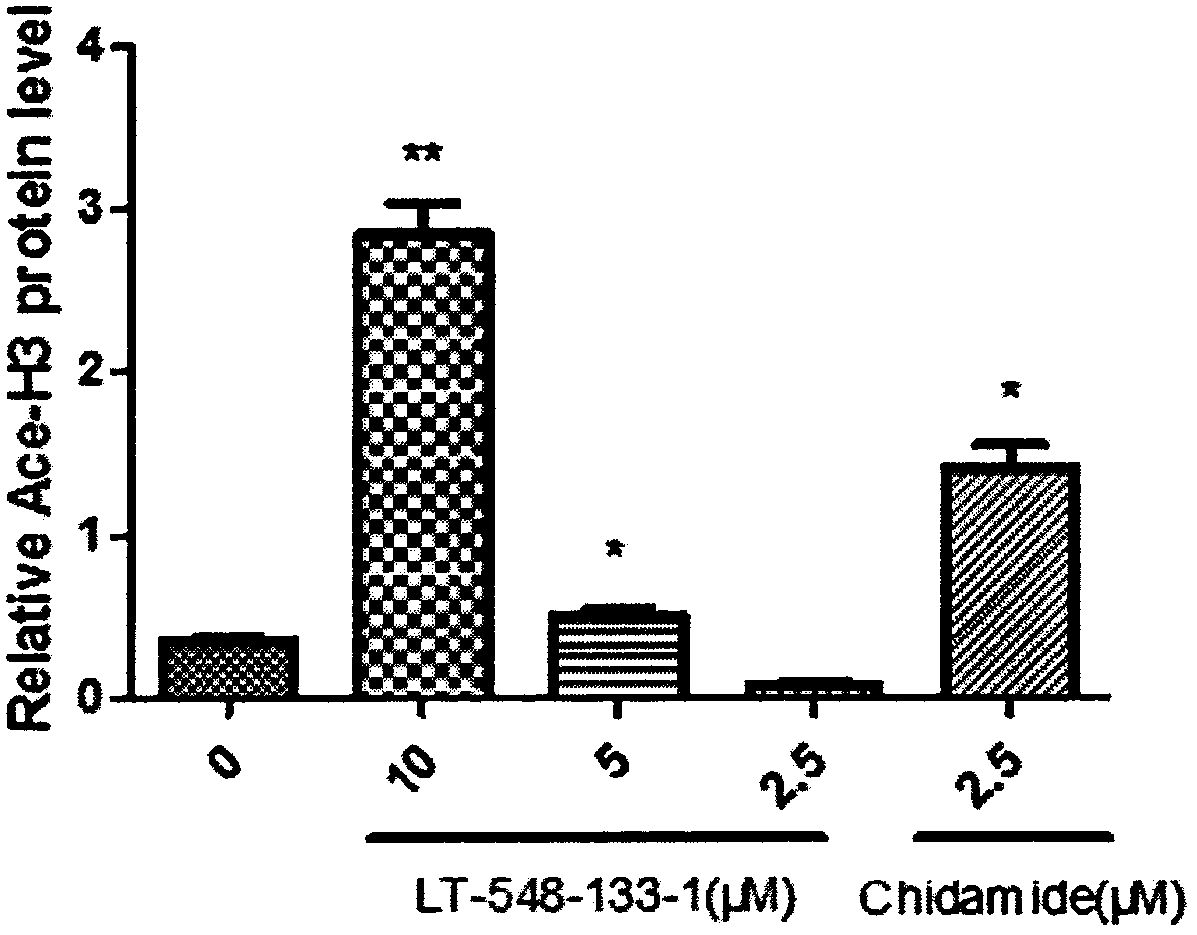
[0016] The effects of LT-548-133-1 and chidamide on the expression of Ace-H3 and p21 of MCF-7 were detected by western blotting:
[0017] MCF-7 cells were cultured in DMEM medium containing 10% fetal bovine serum at 37°C, 5% CO 2 cultured in a cell culture incubator. The medium was changed in 1-2 days, and after the cells were congested, they were digested with 0.25% trypsin and passaged at 1:3. The cells were divided into control group, positive drug (chidamide) group and administration group (10, 5, 2.5 μM). cells in 5 x 10 6 Cells / well were spread in a six-well plate overnight until the cells adhered to the wall, and then administered. After incubation for 24 hours, the cells were digested with 0.25% trypsin to collect the cells. Add 150 μL of RIPA cell lysate to each tube of cells, make the lysate fully contact with the cells, and place on ice for 30 min to lyse. Centrifuge at 12,000 rpm at 4°C for 10 min, and take the supernatant, which is the extracted total protein....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com