Use of collagen implants in the preparation of surgical-assisted restorations for glaucoma
A collagen and implant technology is applied in the field of cowhide purified collagen implants to assist in the field of glaucoma trabeculectomy wound recovery, which can solve the problems of drainage tube displacement, high surgical failure rate, and eye movement disorders.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1. Operation Example of Collagen Implant Assisted Trabeculectomy
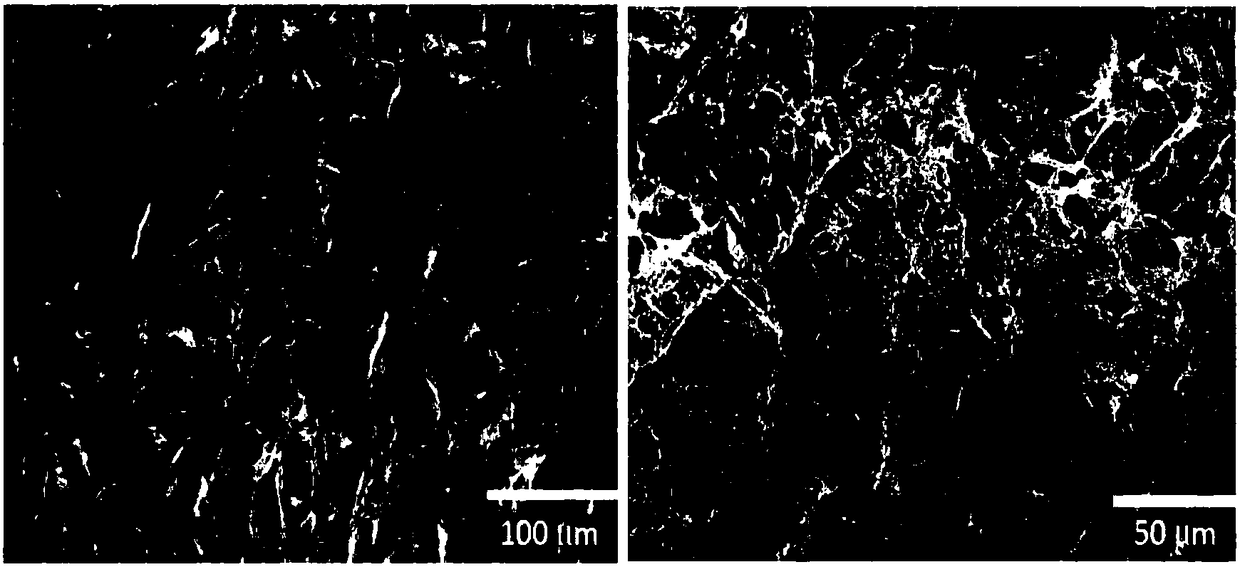
[0032] The collagen implant used as an example in the present invention is completely composed of 100% pure Type I atelocollagen (100% pure Type I Atelocollagen) purified from dried and dehydrated cowhide, with a diameter of 20 to 200 microns three-dimensional holes. Scanning electron microscope observation results enlarged by the appearance of the test piece ( figure 1 ) It can be found that in the dry collagen layered structure, each layer has many micron-scale pores (about 20-200 microns in diameter), and the layers are randomly arranged at intervals of 10-20 microns, And there are many irregular brackets bridging to form holes. Therefore, the three-dimensional structure of the collagen implant of the present invention can be beneficial to the growth of fibroblasts, so that the fibroblasts can grow in the pores of the collagen implant at the initial stage of wound formation, so that A tissu...
Embodiment 2
[0034] Example 2. Endotoxin Content Test of Absorbable Collagen Implants
[0035] Endotoxins are substances produced by Gram-negative bacteria, which can easily trigger allergic and inflammatory reactions in the human body. In order to avoid the impact of endotoxins that may be produced during the manufacturing process of medical devices on the human body, it is necessary to carefully test and evaluate their safety in use. The test system is Limulus amebocyte lysate (LAL). In this example, the endotoxin content of the collagen implant of the present invention was tested by the kinetic coloring method. Endotoxin determination is processed and tested according to the methods and conditions described in ISO10993-12, USP and USP specifications.
[0036] Take 3 test substances (collagen implants), and extract each piece with 5.00mL pyrogen-free water at 37±1°C for 1 hour according to the ratio of 0.2g±10% / mL, and the mixed extract is the test solution. Test substances The test s...
Embodiment 3
[0043] Example 3. Biosafety Testing of Absorbable Collagen Implants
[0044] The composition of medical equipment should consider whether it will cause adverse reactions to the human body. When a medical device is expected to come into contact with human tissue, relevant biocompatibility tests should be carefully performed according to the contact method and time, so as to avoid possible physiological damage caused by the production or contamination of toxic substances during the manufacturing process of the medical device. This example evaluates the possible response of the absorbable collagen implant of the present invention to the muscle of SD rats implanted into the test object 28 days after the muscle implantation stimulation test.
[0045] USP high density polyethylene reference standard (USP high density polyethylene reference standard) (Lot. No. IOK217) was used as a control group. Both the test object (collagen implant of the present invention) and the control object...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com