A kind of preparation method of antifouling cloth for clothing production
An antifouling and cloth technology, applied in the field of fabrics, can solve the problems of loss of moisture absorption, hardening of fabrics, and inability to wash, and achieve the effects of increasing the flexibility of polyester, improving water and oil repellency, and increasing air permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] A preparation method for an antifouling cloth used in clothing production, specifically comprising the following steps:
[0048] Synthesis of S1, myristyl chloride
[0049] The reaction formula is as follows:
[0050]
[0051] Dissolve 120 mol of myristic acid in chloroform, add 600 mol of thionyl chloride, heat up to reflux, and react for 2 hours. After the reaction, concentrate under reduced pressure to remove chloroform and unreacted thionyl chloride to obtain myristyl chloride 2. The yield is 98.6%;
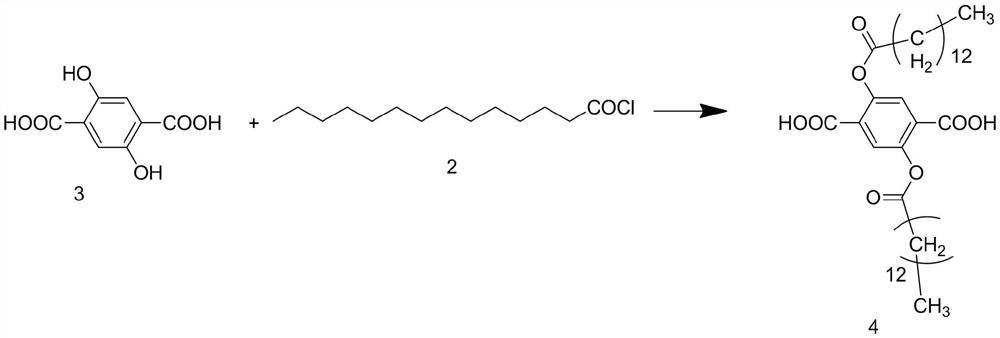
[0052] Synthesis of S2, 2,5-bis(tetradecyl)terephthalic acid
[0053] The reaction formula is as follows:
[0054]
[0055] Dissolve 100 mol of 2,5-dihydroxyterephthalic acid 3 in dichloromethane, add 120 mol of pyridine and stir at room temperature for 15 minutes, then put it in an ice bath, add 120 mol of tetradecanoyl chloride 2 dropwise while stirring, after the dropwise addition is completed, The reaction was stirred for 1 h under ice bath to obtain 2,5-...
Embodiment 2
[0066] A preparation method for an antifouling cloth used in clothing production, specifically comprising the following steps:
[0067] Synthesis of S1, myristyl chloride
[0068] The reaction formula is as follows:
[0069]
[0070] Dissolve 150 mol of myristic acid in chloroform, add 900 mol of thionyl chloride, raise the temperature to reflux, and react for 2 hours. After the reaction, concentrate under reduced pressure to remove chloroform and unreacted thionyl chloride to obtain myristyl chloride 2. The yield is 99.2%;
[0071] Synthesis of S2, 2,5-bis(tetradecyl)terephthalic acid
[0072] The reaction formula is as follows:
[0073]
[0074] Dissolve 100mol 2,5-dihydroxyterephthalic acid 3 in dichloromethane, add 140mol pyridine and stir at room temperature for 15-20min, then put it in an ice bath, add 140mol tetradecanoyl chloride 2 dropwise while stirring, and the dropwise addition is complete Afterwards, the reaction was stirred for 1 h in an ice bath to obt...
Embodiment 3
[0085] A preparation method for an antifouling cloth used in clothing production, specifically comprising the following steps:
[0086] Synthesis of S1, myristyl chloride
[0087] The reaction formula is as follows:
[0088]
[0089] Dissolve 130 mol of myristic acid in chloroform, add 630 mol of thionyl chloride, heat up to reflux, and react for 2.5 hours. After the reaction, concentrate under reduced pressure to remove chloroform and unreacted thionyl chloride to obtain myristyl chloride 2 , the yield is 98.9%;
[0090] Synthesis of S2, 2,5-bis(tetradecyl)terephthalic acid
[0091] The reaction formula is as follows:
[0092]
[0093] Dissolve 100mol of 2,5-dihydroxyterephthalic acid 3 in dichloromethane, add 130mol of pyridine and stir at room temperature for 17min, then put it in an ice bath, add 125mol of tetradecanoyl chloride 2 dropwise while stirring, after the dropwise addition, The reaction was stirred for 1 h under ice bath to obtain 2,5-bis(tetradecyl)ter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation at break | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com