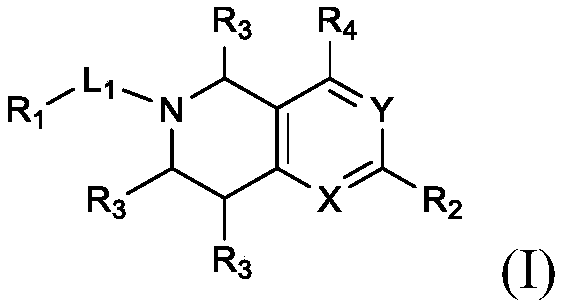
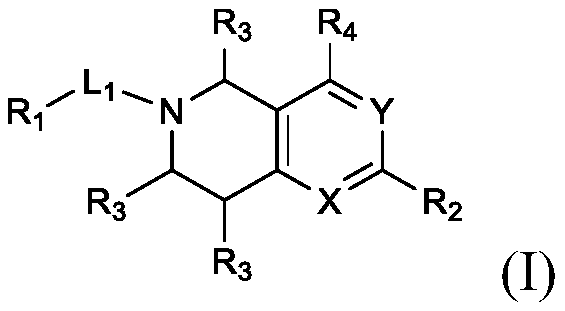
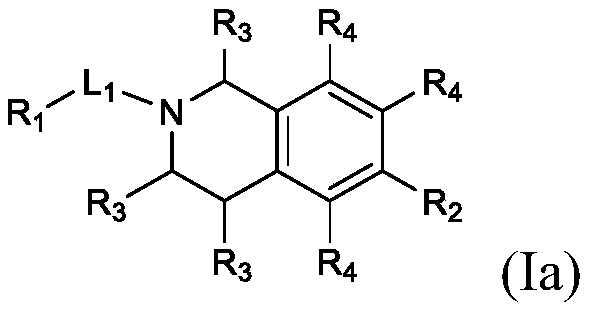
Substituted bicyclic heterocyclic compounds
A technology of compounds and heterocyclic groups, applied in the fields of drug combination, organic chemistry, medical preparations containing active ingredients, etc., can solve problems such as reducing the incidence of hypokalemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-I and 1-II
[0438] 1-(6-(2-Hydroxy-2-(4-methyl-1-oxo-1,3-dihydroisobenzofuran-5-yl)ethyl)-5,6,7,8- Tetrahydropyrido[4,3-d]pyrimidin-2-yl)-1H-indole-4-carbonitrile (enantiomers-I and II)
[0439]
[0440] Intermediate 1A: 1-(6-Benzyl-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidin-2-yl)-1H-indole-4-carbonitrile
[0441]
[0442] K 2 CO 3 (0.798g, 5.78mmol) and XANTPHOS (0.223g, 0.385mmol). The resulting reaction mixture was degassed with nitrogen for 5 min before adding Pd 2 (dba) 3 (0.176 g, 0.193 mmol) and the reaction mixture was degassed with nitrogen for another 5 minutes. The reaction mixture was heated in a sealed tube at 100 °C for 16 hours, cooled and concentrated under reduced pressure. The residue was diluted with EtOAc and filtered through celite. The filtrate was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude solid was washed with diethyl ether (50 mL) to afford Intermediate 1A (0.600 g, 81.1%) as a light yellow solid. ...
Embodiment 1-I
[0446] Example 1-I: (Enantiomer-I)
[0447] Intermediate 1B (0.0950 g, 0.345 mmol) and Intermediate I-1-I (0.0980 g, 0.518 mmol) were dissolved in ethanol (15 mL) and heated to reflux for 48 hours. The resulting reaction mixture was evaporated to dryness under reduced pressure. By preparative HPLC [Sunfire (250x30ID) 5 microns, mobile phase A: 0.1% HCOOH / water, mobile phase B: ACN, gradient: 10-45% B over 7 minutes, flow rate: 25 mL / min, retention time 12.05, UV 254 nm] to obtain Example 1-I (Enantiomer-I) (0.0800 g, 48.3%) as an off-white solid. 1 H NMR (400MHz, DMSO-d 6 )δppm 2.33(s, 3H), 2.66-2.77(m, 2H), 3.00(t, J=5.2Hz, 4H), 3.84(q, J=8.4Hz, 2H), 5.22(t, J=4.4Hz , 1H), 5.40(d, J=2.8Hz, 3H), 5.44(d, J=4.0Hz, 1H), 6.91(dd, J=0.4Hz, J=3.2Hz, 1H), 7.49(t, J =8.0Hz, 1H), 7.70(t, J=8.0Hz, 1H), 7.74(d, J=3.2Hz, 1H), 8.50(d, J=3.6Hz, 1H), 8.64(s, 1H), 9.06 (d, J=8.4Hz, 1H). LCMS (Method-E): Retention time 2.85, [M+H] 466.2. HPLC (Method-N): Retention time 6.71 min, purity 9...
Embodiment 1-II
[0448] Example 1-II: (Enantiomer-II)
[0449] Example 1-II (enantiomer-II) (0.010 g, 6.28%), which is an off-white solid. 1 H NMR (400MHz, DMSO-d 6 )δppm 2.33(s, 3H), 2.66-2.77(m, 2H), 3.00(t, J=5.2Hz, 4H), 3.84(q, J=8.4Hz, 2H), 5.22(t, J=4.4Hz , 1H), 5.40(d, J=2.8Hz, 3H), 5.44(d, J=4.0Hz, 1H), 6.91(dd, J=0.4Hz, J=3.2Hz, 1H), 7.49(t, J =8.0Hz, 1H), 7.70(t, J=8.0Hz, 1H), 7.74(d, J=3.2Hz, 1H), 8.50(d, J=3.6Hz, 1H), 8.64(s, 1H), 9.06 (d, J=8.4Hz, 1H). LCMS / HPLC (Method A): retention time 2.18, [M+1] 466.1, purity: 96.3%. (Method B): retention time 1.39, [M+1] 466.1, purity: 94.5%. Chiral purity (Method XVI): retention time 11.37 min, 98.4% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com