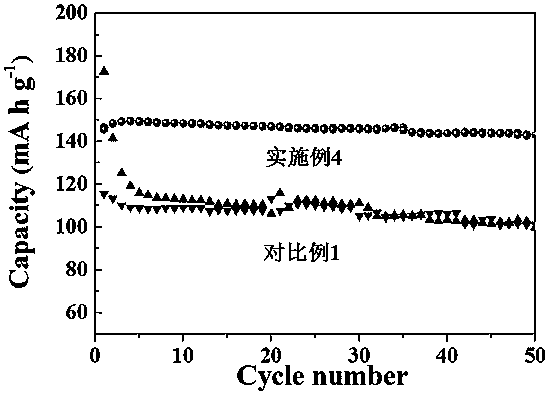
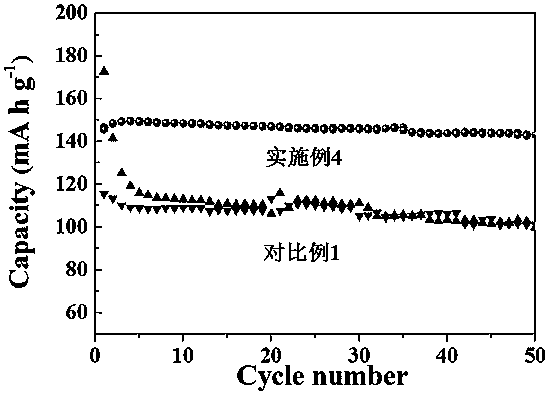
Preparation method and application of a crown ether modified polyaniline solid electrolyte membrane
A technology of solid electrolyte membrane and polyaniline, which is applied in the direction of solid electrolyte, electrolyte battery manufacturing, non-aqueous electrolyte, etc., can solve the problems of high contact resistance and low ion conductivity, and achieve high ion conductivity and good electrochemical performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
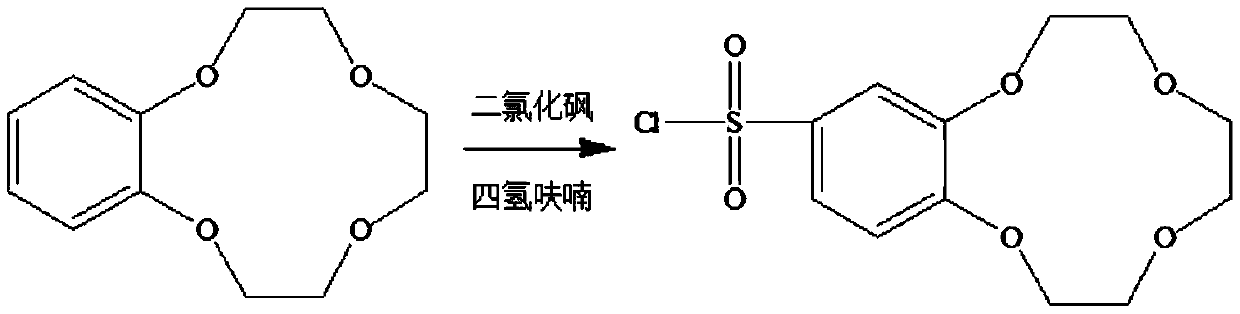
[0021] (1) Preparation of benzo-12-crown-4sulfonyl chloride
[0022] Dissolve 2.24g of benzo-12-crown-4 (0.01mol) in 50mL of tetrahydrofuran, then add it to a 100mL three-necked round-bottomed flask equipped with a magnet, reflux condenser and thermometer, pass into nitrogen protection and react After cooling the system to 0°C, add 8 mL of freshly distilled sulfone dichloride (0.1 mol) dropwise into the round-bottomed flask with a glass syringe, and the dropping time is not less than 30 min. After the dropwise addition, the temperature was raised to 35°C for 6 hours. After the reaction, the mixture was dried and neutralized with quicklime, and the tetrahydrofuran was removed by vacuum rotary evaporation. The remaining oil was recrystallized in n-hexane and placed in a vacuum oven at 70°C for vacuum drying. After 24 hours or more, the obtained white solid powder was benzo-12-crown-4sulfonyl chloride (2.11 g, 0.00655 mol), and the yield was 65.5%. Its reaction equation is shown...
Embodiment 2
[0036] (1) Preparation of benzo-14-crown-4sulfonyl chloride
[0037] Dissolve 2.52g of benzo-14-crown-4 (0.01mol) in 70mL of ethyl acetate, then add it into a 250mL three-necked round-bottomed flask equipped with a magnet, reflux condenser and thermometer, pass into nitrogen protection and After the reaction system was cooled to 0°C, 10 mL of freshly distilled chlorosulfonic acid (0.15 mol) was added dropwise into the round bottom flask with a glass syringe, and the dropping time was not less than 30 min. After the dropwise addition, the temperature was raised to 45°C for 7 hours. After the reaction, the mixture was dried and neutralized with caustic soda, and the ethyl acetate was removed by vacuum rotary evaporation. The remaining oil was recrystallized in n-heptane and placed in a vacuum oven at 100°C. After vacuum drying in the oven for more than 48 hours, the obtained white solid powder was benzo-14-crown-4sulfonyl chloride (2.1 g, 0.006 mol), with a yield of 60%. Its re...
Embodiment 3
[0051] (1) Preparation of dibenzo-12-crown-4sulfonyl chloride
[0052] Dissolve 2.72g of benzo-12-crown-4 (0.01mol) in 50mL of tetrahydrofuran, then add it to a 100mL three-necked round-bottomed flask equipped with a magnet, reflux condenser and thermometer, pass into nitrogen protection and react After cooling the system to 0°C, add 16 mL of freshly distilled sulfone dichloride (0.2 mol) dropwise into the round-bottomed flask with a glass syringe, and the dropping time is not less than 30 min. After the dropwise addition, the temperature was raised to 55°C for 8 hours. After the reaction, the mixture was dried and neutralized with quicklime, and the tetrahydrofuran was removed by vacuum rotary evaporation. The remaining oil was recrystallized in n-hexane and placed in a vacuum oven at 70°C for vacuum drying. After 24 hours, the obtained yellow solid powder was dibenzo-12-crown-4sulfonyl chloride (2.88 g, 0.00615 mol), and the yield was 61.5%. Its reaction equation is shown i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com