Immunologic effector cell targeting CPC3 and application thereof
A technology of immune response cells and cells, applied in blood/immune system cells, genetically modified cells, applications, etc., can solve problems such as death, weight loss, and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Example 1. Preparation of GPC3-CAR-T cells / IL12-GPC3-CAR T cells
[0182] 1. Plasmid construction
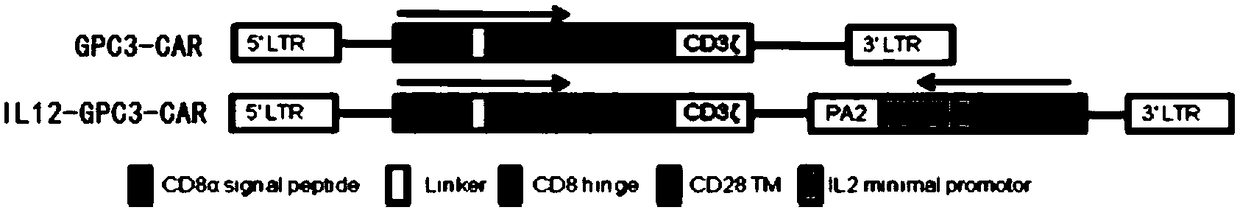
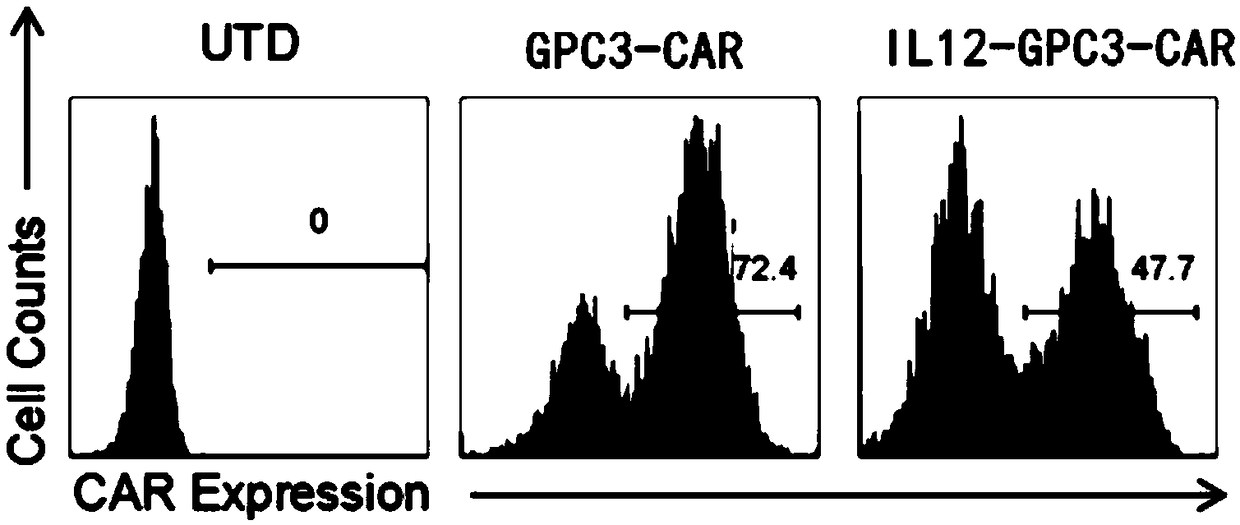
[0183] The chimeric antigen receptor used in this example is a second-generation chimeric antigen receptor, and the nucleotide coding sequence of the scFv targeting the extracellular domain of the GPC3 receptor is shown in SEQ ID NO: 1, Also has the transmembrane domain of CD28, the intracellular domain of CD28, and CD3ζ. refer to Figure 1A As indicated, construct the plasmids of GPC3-CAR-T cells and IL12-GPC3-CAR T cells, respectively, as follows:
[0184] The GPC3-CAR-T sequence consists of CD8α signal peptide (SEQ ID NO: 2), scFv targeting GPC3 (SEQ ID NO: 1), CD8 hinge region (SEQ ID NO: 3), CD28 transmembrane region (SEQ ID NO: 6) and the intracellular signaling domain (SEQ ID NO: 4) and the intracellular segment of CD3 CD3ζ (SEQ ID NO: 5).
[0185] The IL12-GPC3-CAR plasmid is based on the GPC3-CAR-T plasmid with the NFAT6-IL12 sequence inserted, and a second-ge...
Embodiment 2
[0205] Example 2, Cytotoxicity assay targeting GPC3CAR-T / IL12-GPC3-CAR T cells
[0206] The CytoTox 96 non-radioactive cytotoxicity detection kit (Promega Company) was used to detect cytotoxicity, specifically referring to the instructions of the CytoTox 96 non-radioactive cytotoxicity detection kit.
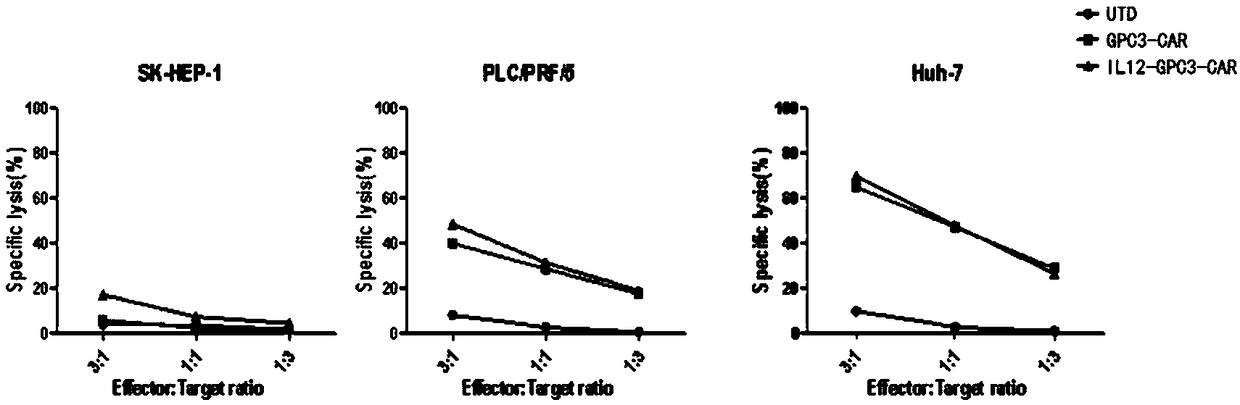
[0207] 1) Target cells: select Huh-7, PLC / PRF / 5, and SK-HEP-1 cells as target cells, wherein Huh-7 cells, PLC / PRF / 5 cells are GPC3 positive, and SK-HEP-1 cells are GPC3 negative;
[0208] 2) Effector cells: add UTD, GPC3-CAR-T and IL12-GPC3-CAR T cells according to the effect-to-target ratio of 3:1, 1:1 or 1:3;
[0209] Experimental results such as figure 2 As shown, compared with the UTD group, GPC3-CAR-T / IL12-GPC3-CAR T showed strong cytotoxicity in the in vitro toxicity test, and there was no significant difference between the two. For GPC3-negative liver cancer cells, GPC3-CAR-T / IL12-GPC3-CAR T did not enhance the killing effect.
Embodiment 3
[0210] Example 3, GPC3-CAR-T / IL12-GPC3-CAR T cytokine secretion
[0211] UTD / GPC3-CAR-T / IL12-GPC3-CAR T was co-incubated with liver cancer cell lines Huh-7, PLC / PRF / 5, SK-HEP-1 cells at a ratio of 1:1 for 24 hours, then the supernatant was collected, and the supernatant was detected by ELISA Cytokine secretion levels.
[0212] The samples for detecting TNF-α and IL12 do not need to be diluted, and the samples for detecting IL2 and IFN-γ are diluted 20 times and 25 times respectively. The ELISA kit adopts double-antibody sandwich enzyme-linked immunosorbent detection technology. The results are shown in Figures 3A and 3B. Figure 3A It shows that IL12-GPC3-CAR T has a higher level of IL12 secretion when co-incubating with GPC3+ tumor cells Huh-7 and PLC / PRF / 5, and almost IL12 could not be detected, indicating that the CAR-T cells could secrete IL12 only when GPC3 was recognized. Figure 3B In order to detect the release of cytokines IL-2, TNF-α, and IFN-γ when UTD / GPC3-CAR-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com