Anti-repulsive guidance molecule a (RGMA) antagonistic antibodies for treating spinal cord injury and pain
A spinal cord injury and antibody technology, applied in the direction of antibodies, antibody medical components, anti-growth factor immunoglobulin, etc., can solve problems such as not representative
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 11
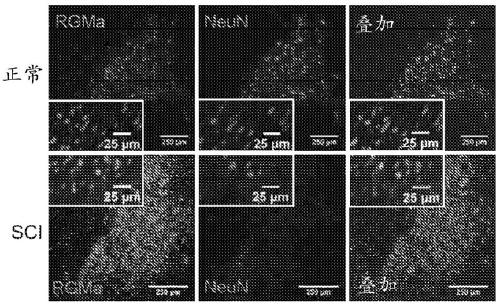
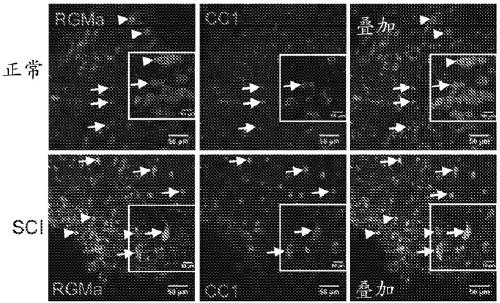
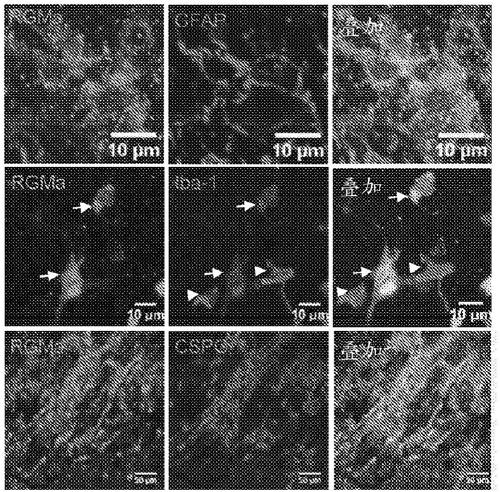
[0180] RGMa expression in rat and human spinal cord after SCI
[0181] Methodology: Rat tissue sections were prepared for immunohistochemical staining and incubated overnight at 4°C with primary antibodies. The following primary antibodies were used: NeuN for neurons (1:500, Millipore Bioscience Research Reagents), GFAP for astrocytes (1:200, Millipore), activated giant Iba-1 for phagocytes / microglia (1:1000, and Wako Chemicals), CS56 for chondroitin sulfate proteoglycan (1:500, Sigma), calcitonin for sensory fibers CGRP (1:1000, Millipore), 5HT for serotonergic fibers (1:3000, ImmunoStar), and hIgG for detecting human IgG antibodies (1:500, Millipore Inc. (Millipore). Sections were incubated overnight with primary antibodies diluted in blocking solution, washed, and incubated with fluorescently-conjugated secondary antibodies.
[0182] Human tissue sections were prepared for staining and incubated overnight with primary antibodies (1 :200 RGMa, Abcam; or 1 :100 Regenerate, ...
example 12
[0186] Effect of anti-RGMa antibody on neurite outgrowth in vitro
[0187] Methodology: For neurite outgrowth assays, E18 mouse cortical neurons were plated on poly-L-lysine treated with laminin (Invitrogen; 10 mg / ml) and RGMa protein (5 mg / ml). Amino acid-coated glass coverslips and incubated with control antibody (hIgG) or anti-RGMa (1 mg / ml) for 24 hours at 37°C. For neurite outgrowth analysis, cortical cells were immunostained with βIII-tubulin (Sigma; 1:500). For western blotting, mouse cortical neurons were lysed in RIPA buffer and loaded onto 10% acrylamide gels, then transferred to nitrocellulose membranes. Blots were probed with anti-RGMa antibodies (AE12-1 and AE12-1-Y) and anti-regenerate protein (E20; Santa Cruz; 10 mg / ml).
[0188] Results: In Western blot of mouse cortical neuron lysates, both AE12-1 and AE12-1-Y specifically detected a 50kDa band ( Figure 3A ). Cultured mouse primary cortical neurons were also shown to express RGMa, as shown by immunostaini...
example 13
[0190] Detection of anti-RGMa antibodies in serum, CSF, and spinal cord
[0191] Methodology: Cerebrospinal fluid (CSF) was sampled via lumbar puncture (LP) 6 weeks after SCI. Nine weeks after SCI, 3 weeks after the last antibody administration, serum was collected and rats were perfused. Antibody concentrations in CSF and serum samples from AE12-1, AE12-1-Y, and hlgG-treated rats were determined using ELISA assays.
[0192] Results: Antibody concentrations in the CSF of rats injected with AE12-1 ranged from 0.25-8.20 μg / ml, and for AE12-1-Y, antibody concentrations ranged from 0.33-6.77 μg / ml ( Figure 4B ). In contrast, antibody concentrations were considerably higher in serum, approximately 10-fold greater than in CSF, as antibodies were injected intravenously after the initial intraspinal injection immediately after SCI ( Figure 4C ). In addition, antibody concentrations continued to rise in serum 3 weeks after the last dose. Human antibodies were detected in the inj...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com