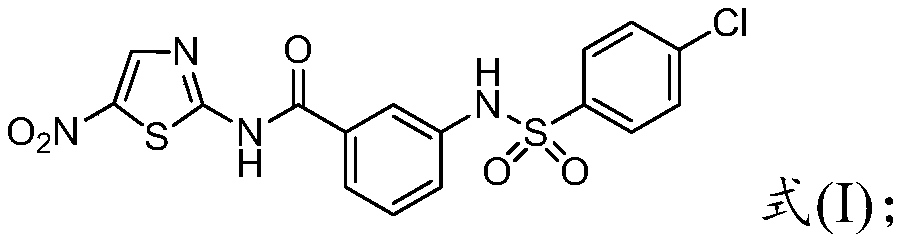
An anti-caries and anti-bacterial thiazole compound and its preparation method
A technology of compounds and compound structural formulas, applied in the field of medicine, can solve problems such as difficulty in maintaining long-acting and effective antibacterial effects, and achieve the effect of reducing growth vitality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
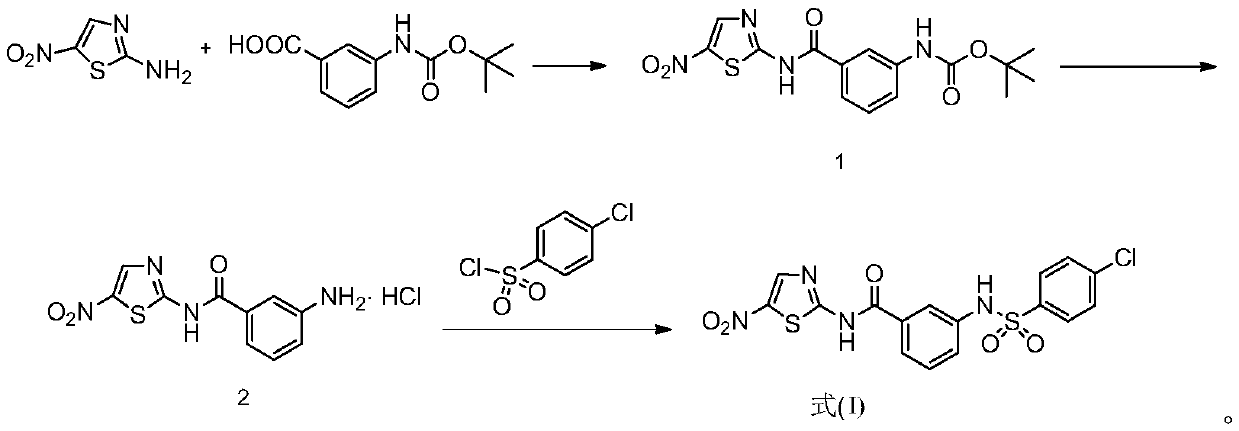
[0056] The preparation of embodiment 1 formula (I) compound
[0057] The synthetic route is:
[0058]
[0059] Reagents and reaction conditions used in the synthetic route: (a) EDCI, DMAP, anhydrous N,N-dimethylformamide, room temperature; (b) HCl / ethyl acetate saturated solution, room temperature, 15min; (c) carbonic acid Potassium, dioxane / water (V:V=1:1), room temperature, 30min.
[0060] Concrete preparation process comprises the following steps:
[0061] (1) Preparation of (3-((5-nitrothiazol-2-yl)carbamoyl)phenyl)carbamate tert-butyl ester (intermediate 1)
[0062] Dissolve 2-amino-5-nitrothiazole (1mmol) and 3-(Boc-amino)benzoic acid (1.2mmol) in 12mL of anhydrous N,N-dimethylformamide, then add EDCI (2mmol) and DMAP (2mmol), reacted at room temperature for 12h, added 30mL of ethyl acetate to the reaction solution for dilution, washed twice with 1mol / L HCl aqueous solution (2×30mL), washed twice with saturated sodium bicarbonate solution (1 × 30 mL), and then w...
Embodiment 2
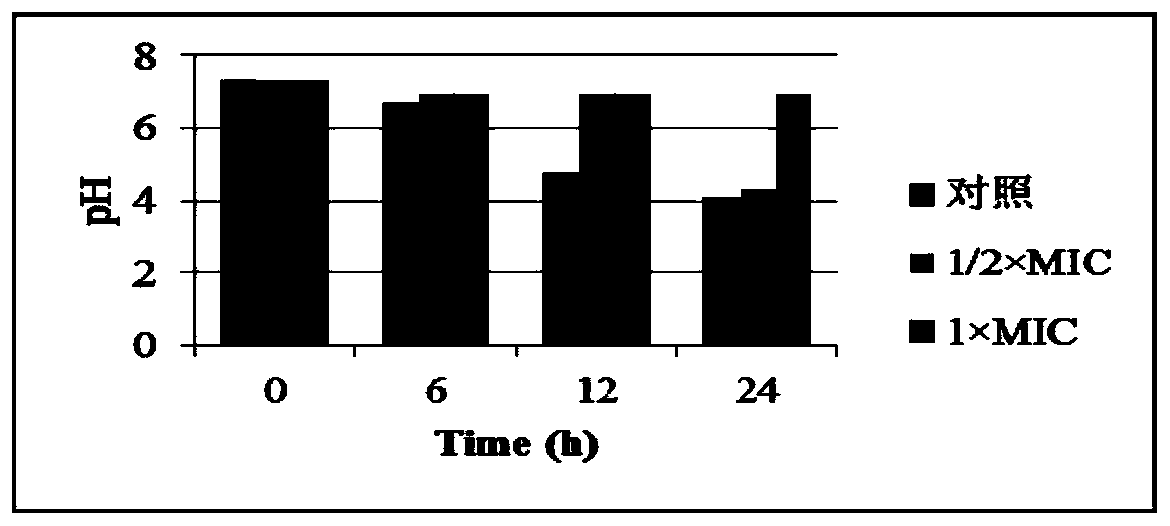
[0067] Embodiment 2. Antibacterial activity experiment of formula (I) compound
[0068] 1. Preparation of Streptococcus mutans UA159 strain (S.mutans UA159) and formula (I) compound
[0069] The Streptococcus mutans UA159 strain used in the present invention is a type strain, and the reference genome number in the NCBI database (http: / / www.ncbi.nlm.nih.gov / ) is NC_004350. The Streptococcus mutans UA246 strain used in the present invention is a clinical strain isolated from the oral cavity of a caries patient. The most suitable culture medium is preferably BrainHeart Infusion medium (Brain infusion solids 12.5g / L, Beef heart infusion solids 5.0g / L, Proteose peptone 10.0g / L, Glucose 2.0g / L, Sodium chloride 5.0 g / L, Di-sodium phosphate 2.5g / L, pH 7.4±0.2), static culture at 37°C, the most suitable condition for anaerobic culture.
[0070] (1) The medium for cultivating Streptococcus mutans is Brain Heart Infusion medium (brand OXOID, product number CM1135). The main component...
Embodiment 3
[0085] Embodiment 3. The effect experiment of formula (I) compound on oral cavity normal bacteria
[0086] The experimental procedure was the same as in Example 2, and the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the compound on normal oral bacteria L.delbrueckii CICC 6032, V.denticariosi KQ-ETV-9, and V.rogosae WZH3n were tested. The results are shown in Table 2.
[0087] Table 2. Antibacterial and bactericidal activity results (unit μg / mL) of the compound of formula (I) to normal oral bacteria
[0088]
[0089] It can be seen from Table 2 that the compound of formula (I) has no effect on normal oral bacteria, which is even smaller than the positive control drug chlorhexidine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com