Fluorescent biosensor for detecting kanamycin and preparation method and application thereof
A biosensor, kanamycin technology, applied in the field of biosensors, can solve the problems of cumbersome sample pretreatment or concentration process, cumbersome operation, difficult to popularize, etc., and achieve the effects of fast detection speed, good repeatability and low detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] A method for preparing a fluorescent biosensor for detecting kanamycin, comprising the following steps:
[0061] (1) Preparation of gold nanoparticles;
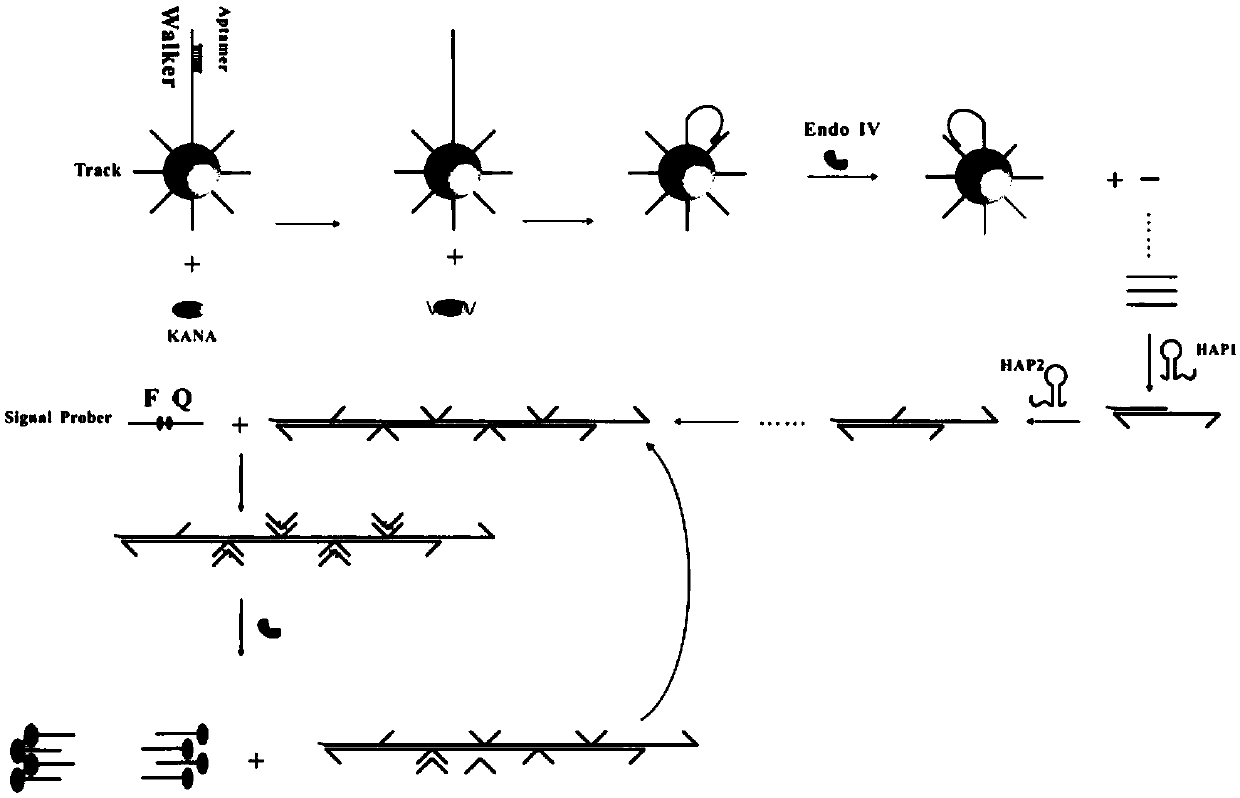
[0062] (2) Preparation of modified gold nanoparticles solution: modify Aptamer, Walker and Track on the surface of gold nanoparticles;
[0063] (3) Hybrid chain reaction: Mix the modified gold nanometer solution with the homogeneous reaction solution;
[0064] (4) Fluorescence detection.
[0065] In the step (1), the preferred preparation steps of gold nanoparticles are as follows:
[0066] (1) Install the required instruments and add 200mL ultrapure water into the three-necked flask (be careful not to let dust fall into the three-necked flask).
[0067] (2) Take 500uL (0.04g / mL) HAuCl4 in a single-package centrifuge tube, use a pipette gun to take 500uL and 200mL ultrapure water, stir and heat at a stirring speed of about 450 rpm, until boiled.
[0068] (3) Under the condition of stirring, take 3mL of 1% trisodium...
Embodiment 2
[0084] A method for preparing a fluorescent biosensor for detecting kanamycin, comprising the following steps:
[0085] (1) Preparation of gold nanoparticles;
[0086] (2) Preparation of modified gold nanoparticles solution: modify Aptamer, Walker and Track on the surface of gold nanoparticles;
[0087] (3) Hybrid chain reaction: Mix the modified gold nanometer solution with the homogeneous reaction solution;
[0088] (4) Fluorescence detection.
[0089] Steps (1), (2), and (4) are the same as in Example 1.
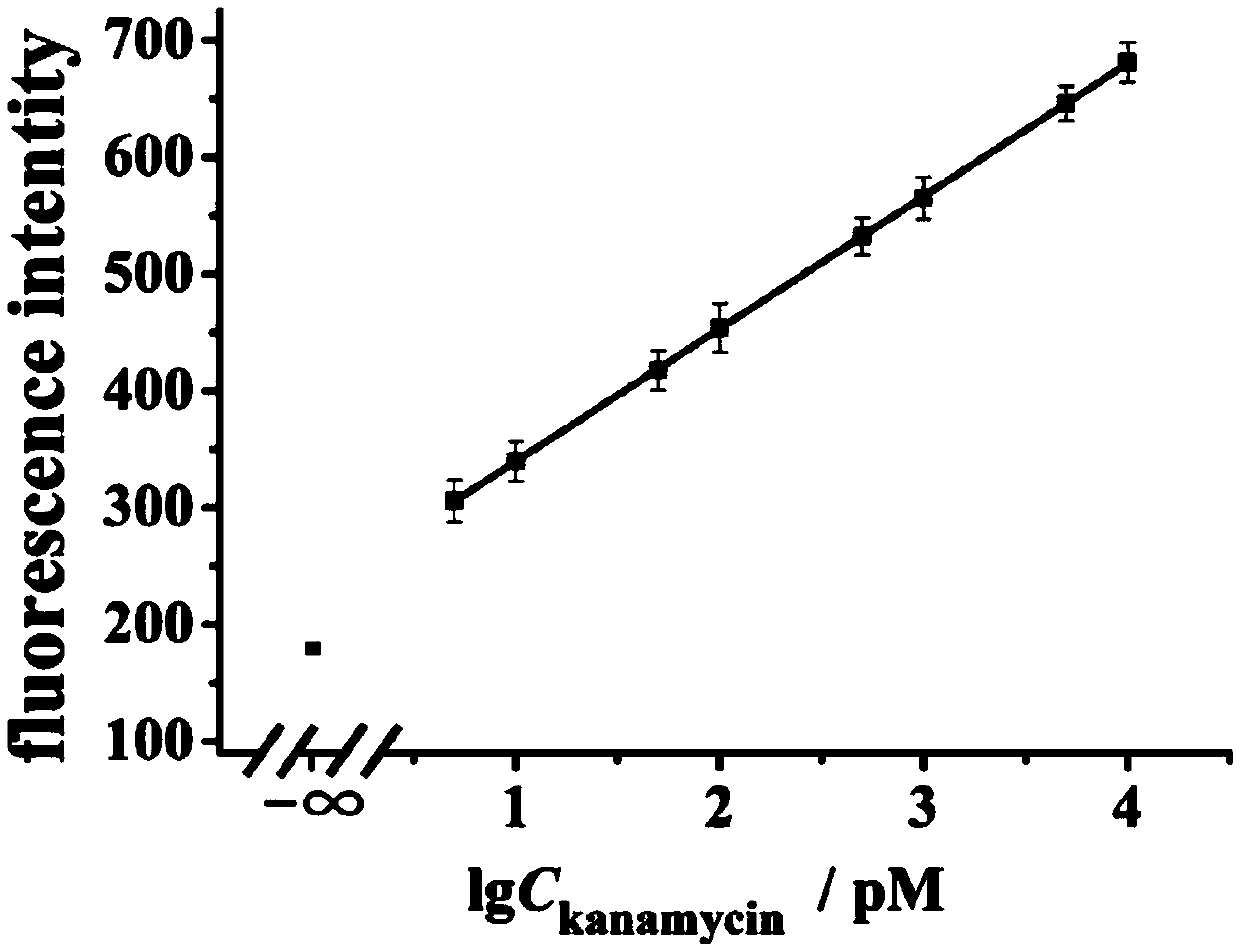
[0090] The main steps of the reaction process of step (3) are as follows: sterilized water, target substance (4 μL), modified nano-gold solution (6 μL), HAP1 (2 μL) (final concentrations were 0.1 nM, 0.2 nM, 0.3nM, 0.4nM, 0.5nM, 0.6nM), 10× buffer (buffer) (4 μL), restriction endonuclease IV (3 μL), signal probe (6 μL), added to In pre-prepared sterile EP tubes. Shake for 30 s and incubate for 2 h in a 37°C incubator.
[0091] The excitation wavelength of the fluore...
Embodiment 3
[0094] A method for preparing a fluorescent biosensor for detecting kanamycin, comprising the following steps:
[0095] (1) Preparation of gold nanoparticles;
[0096] (2) Preparation of modified gold nanoparticles solution: modify Aptamer, Walker and Track on the surface of gold nanoparticles;
[0097] (3) Hybrid chain reaction: Mix the modified gold nanometer solution with the homogeneous reaction solution;
[0098] (4) Fluorescence detection.
[0099] Steps (1), (2), and (4) are the same as in Example 1.
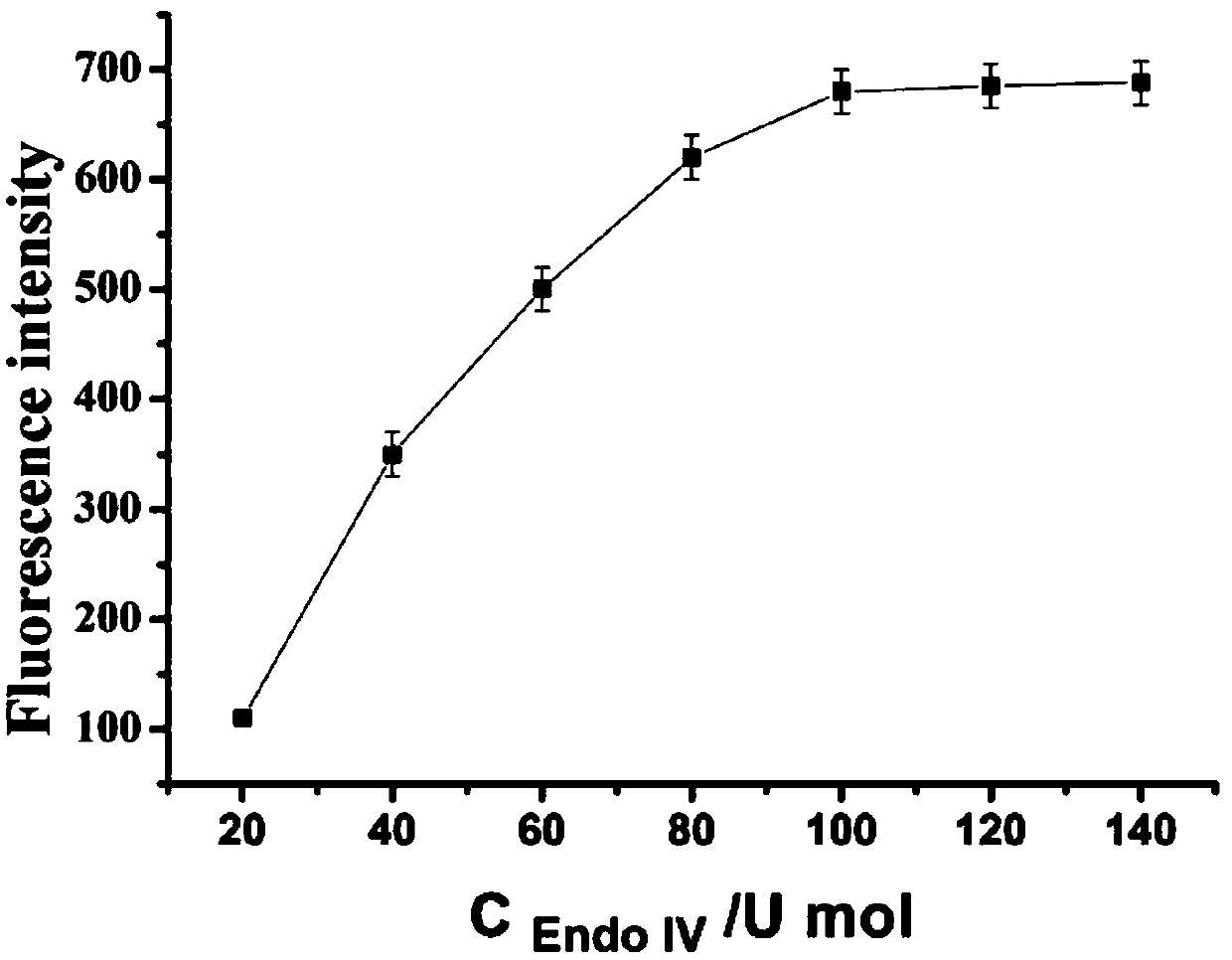
[0100] The main steps of the reaction process in step (3) are as follows: sterilized water, target substance (4 μL), modified nano-gold solution (6 μL), HAP1 (2 μL), HAP2 (2 μL), 10× buffer (3 μL), restriction endonuclease IV (3 μL), signal probe (6 μL) (final concentrations were 0.2 μM, 0.4 μM, 0.6 μM, 0.8 μM, 1.0 μM, 1.2μM, 1.4μM), added to the pre-prepared sterilized EP tube. Shake for 30 s and incubate for 2 h in a 37°C incubator.
[0101] The excitation wavelen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Final concentration | aaaaa | aaaaa |
| Final concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com