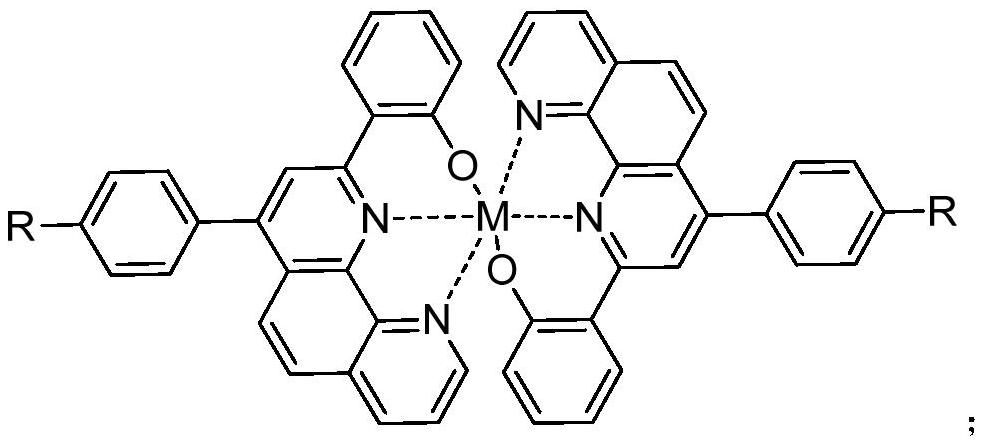
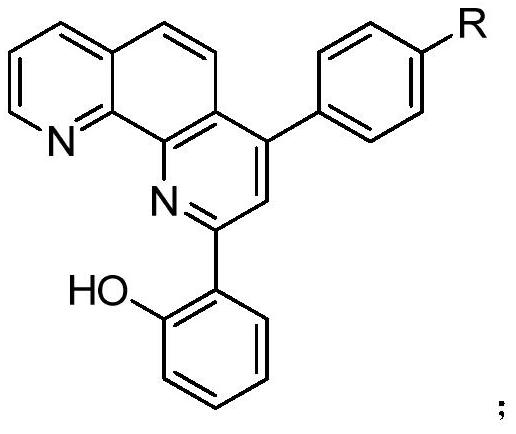
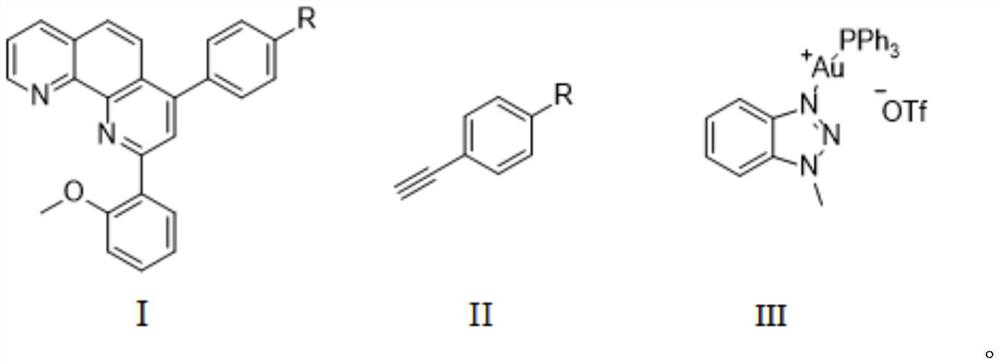
1,10-phenanthroline derivative metal-organic complex and preparation method thereof
A metal organic and derivative technology, applied in 1 field, can solve the problems of difficult synthesis of catalysts, single synthesis raw materials, long synthesis steps, etc., and achieve the effects of simple synthesis method, high fluorescence activity, and easily available raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1:1,10-phenanthroline derivative ligand L 1 Preparation of:
[0030]
[0031] Pour 8-aminoquinoline (0.288g, 2mmol) and o-methoxybenzaldehyde (0.3g, 2.2mmol) into a dry round-bottomed flask in turn, stir evenly, and heat to 60°C for 2h. After cooling down, add 20mL of dry toluene to the reaction system to disperse the reactants evenly, then add phenylacetylene (0.224g, 2.2mmol) and TA-Au (70mg, 0.1mmol) successively under nitrogen atmosphere, stir at room temperature for 10min and then heat To reflux, 36h. After the reaction was complete, it was directly spin-dried and passed through the column to obtain a light gray solid with a yield of 38%.
[0032] Under nitrogen protection, the above product (2-(2-methoxyphenyl)-4-phenyl-1,10-phenanthroline) (1.10g, 3mmol) and sodium hydroxide (0.36g, 9mmol, 3eq) was successively added to a round bottom flask filled with 5mL NMP (N-methylpyrrolidone), and after complete dissolution, DodSH (n-dodecanethiol) (0.91g, ...
Embodiment 2
[0033] Embodiment 2: 1,10-phenanthroline derivative ligand L 2 Preparation of:
[0034]
[0035] Pour 8-aminoquinoline (0.288g, 2mmol) and o-methoxybenzaldehyde (0.3g, 2.2mmol) into a dry round-bottomed flask in turn, stir evenly, and heat to 60°C for 2h. After cooling down, 20mL of dry toluene was added to the reaction system to uniformly disperse the reactants, and A (0.232g, 2.2mmol) and TA-Au (70mg, 0.1mmol) were added successively under a nitrogen atmosphere, stirred at room temperature for 10min and then heated to Reflux, 36h. After the reaction was complete, it was directly spin-dried and passed through the column to obtain a light gray solid with a yield of 45%.
[0036] Under nitrogen protection, the previous step product (1.12g, 3mmol), sodium hydroxide (0.36g, 9mmol, 3eq) were successively added in a round bottom flask containing 5mL NMP, and DodSH (0.91g, 4.5mmol, 1.5eq), and heated at 130 ° C for 3h. After the reaction was complete, the reaction temperature...
Embodiment 3
[0037] Embodiment 3: 1,10-phenanthroline derivative ligand L 3 preparation.
[0038]
[0039] Pour 8-aminoquinoline (0.288g, 2mmol) and o-methoxybenzaldehyde (0.3g, 2.2mmol) into a dry round-bottomed flask in turn, stir evenly, and heat to 60°C for 2h. After cooling down, 20 mL of dry toluene was added to the reaction system to uniformly disperse the reactants, and then added B (0.254 g, 2.2 mmol) and TA-Au (70 mg, 0.1 mmol) successively under a nitrogen atmosphere, stirred at room temperature for 10 min, and then heated to Reflux, 36h. After the reaction was complete, it was directly spin-dried and passed through the column to obtain a light gray solid with a yield of 43%.
[0040]Under nitrogen protection, the previous step product (1.16g, 3mmol), sodium hydroxide (0.36g, 9mmol, 3eq) were successively added in a round bottom flask filled with 5mL NMP, and DodSH (0.91g, 4.5mmol, 1.5eq), and heated at 130 ° C for 3h. After the reaction was complete, the reaction tempera...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com