Application of HLA-A*24:02 allele to detecting person drug eruptionrisk caused by metronidazole
An HLA-A, allele technology, applied in the field of biomedicine, can solve the problems of high mortality and morbidity, severe morbidity, personal and social burden, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
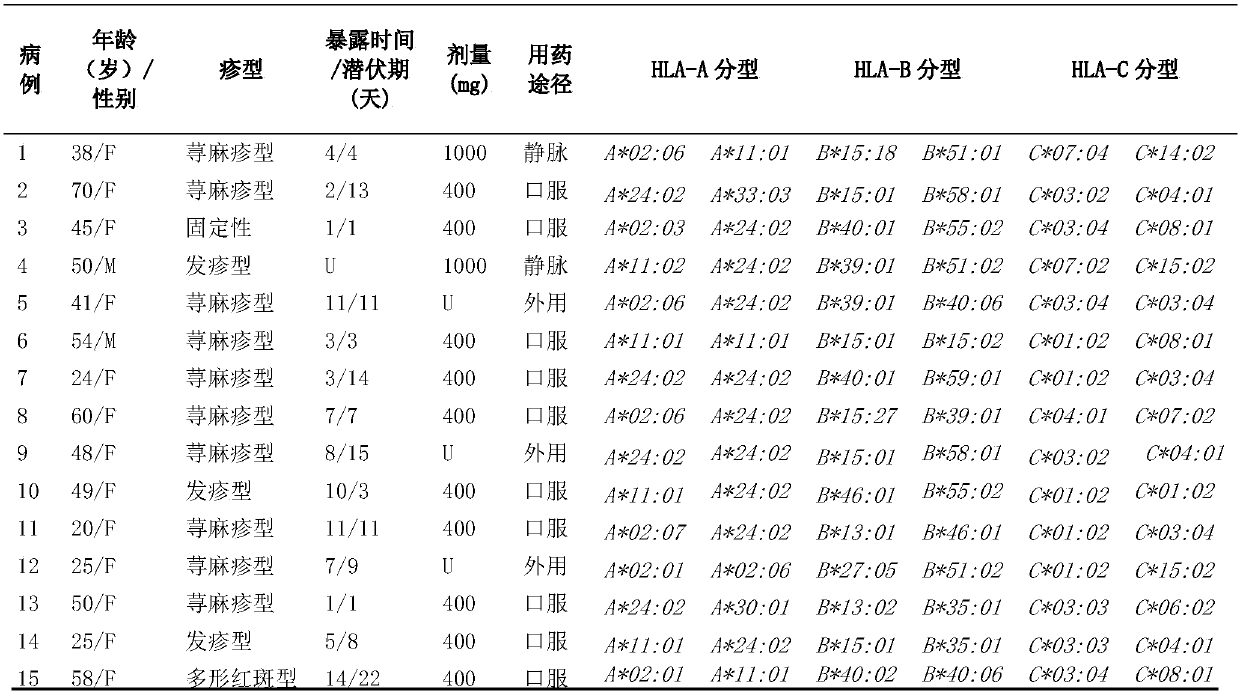
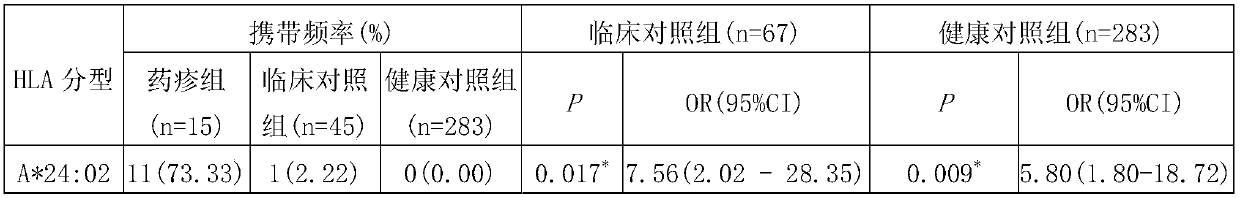
[0030] Example 1. HLA-A*24:02 allele is a risk genetic marker for drug eruption caused by metronidazole
[0031] 1. Sample
[0032] The samples were human peripheral blood, including samples of metronidazole-induced drug eruption, samples of metronidazole-resistant patients, and samples of healthy controls.
[0033] 1. Drug eruption group caused by metronidazole
[0034] 15 patients with drug eruption caused by metronidazole (drug eruption group); the average age of the patients was 43.80±14.84 years old, the ratio of male to female was 2:13; the incubation period of metronidazole was 7.21±5.07 days (1-15 days). The main way for patients to use metronidazole was oral administration (10 cases), followed by external application (3 cases), and intravenous injection (2 cases).
[0035] The clinical manifestations of the patients were ordinary drug eruption, among which 10 cases were urticarial drug eruption, showing generalized wheal-like rash, which was difficult to subside wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com