Indium compound with 2,6-diacetylpyridine thiosemicarbazone as ligand and synthesis method and application of indium compound
A technology of diacetylpyridine thiosemicarbazone and diacetylpyridine, which is used in the synthesis and application of indium compounds, and can solve problems such as large side effects, unclear targets, and inability to realize diagnosis and treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthesis of indium compound C1 comprises the following steps:
[0032] (1) Dissolve 2,6-diacetylpyridine (0.815g, 5mmol) in 20mL of ethanol and stir at 60°C for 15min to obtain a solution;
[0033] (2) Add thiosemicarbazide (0.911g, 10mmol) to the prepared solution, reflux and stir the reaction at 60°C for 12h, cool to room temperature after the reaction, pour it into a beaker for volatilization, filter the obtained pale yellow precipitate, and use Washed with water and ethanol for 3 times to obtain ligand L1;
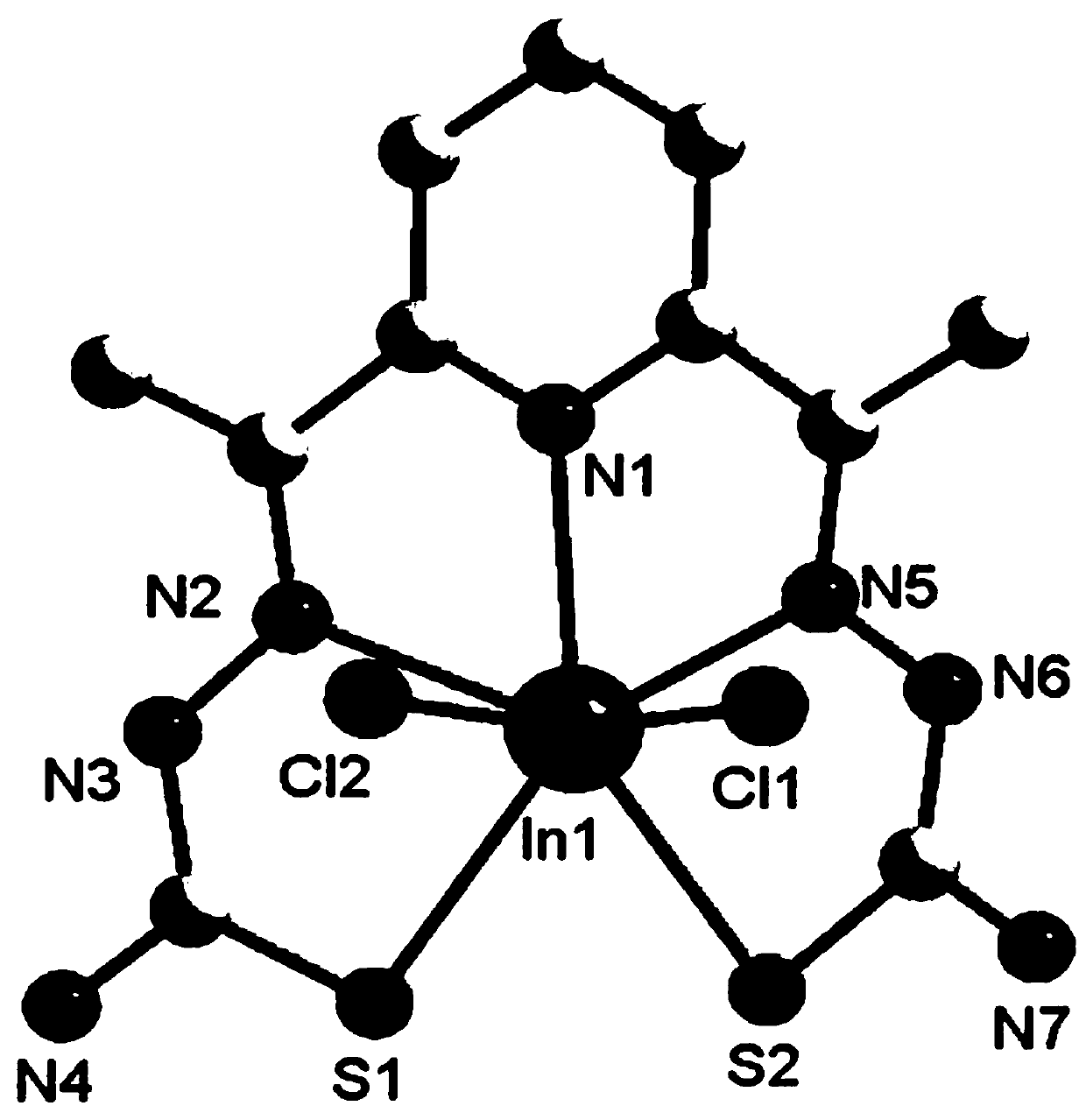
[0034] (3) Take InCl 3 (0.221g, 1mmol) was dissolved in 20mL of methanol solution, and then added dropwise to 10mL ethanol solution containing 1mmol of 2,6-diacetylpyridine thiosemicarbazone ligand, and stirred at 60°C for 6h under reflux. The solution was filtered into a 50mL beaker, sealed with plastic wrap, needled into 6 holes, and volatilized at room temperature for several days to obtain pale yellow crystal C1 with a single crystal structure as fig...
Embodiment 2
[0037] The synthesis of indium compound C2 comprises the following steps:
[0038] (1) Dissolve 2,6-diacetylpyridine (0.815g, 5mmol) in 20mL of ethanol and stir at 60°C for 15min to obtain a solution;
[0039] (2) Add 4-methylthiosemicarbazide (1.05g, 10mmol) to the prepared solution, reflux at 60°C and stir for 12h. After the reaction, cool to room temperature and pour it into a beaker for volatilization. Filter the obtained pale yellow precipitate , washed 3 times with absolute ethanol to obtain ligand L2;
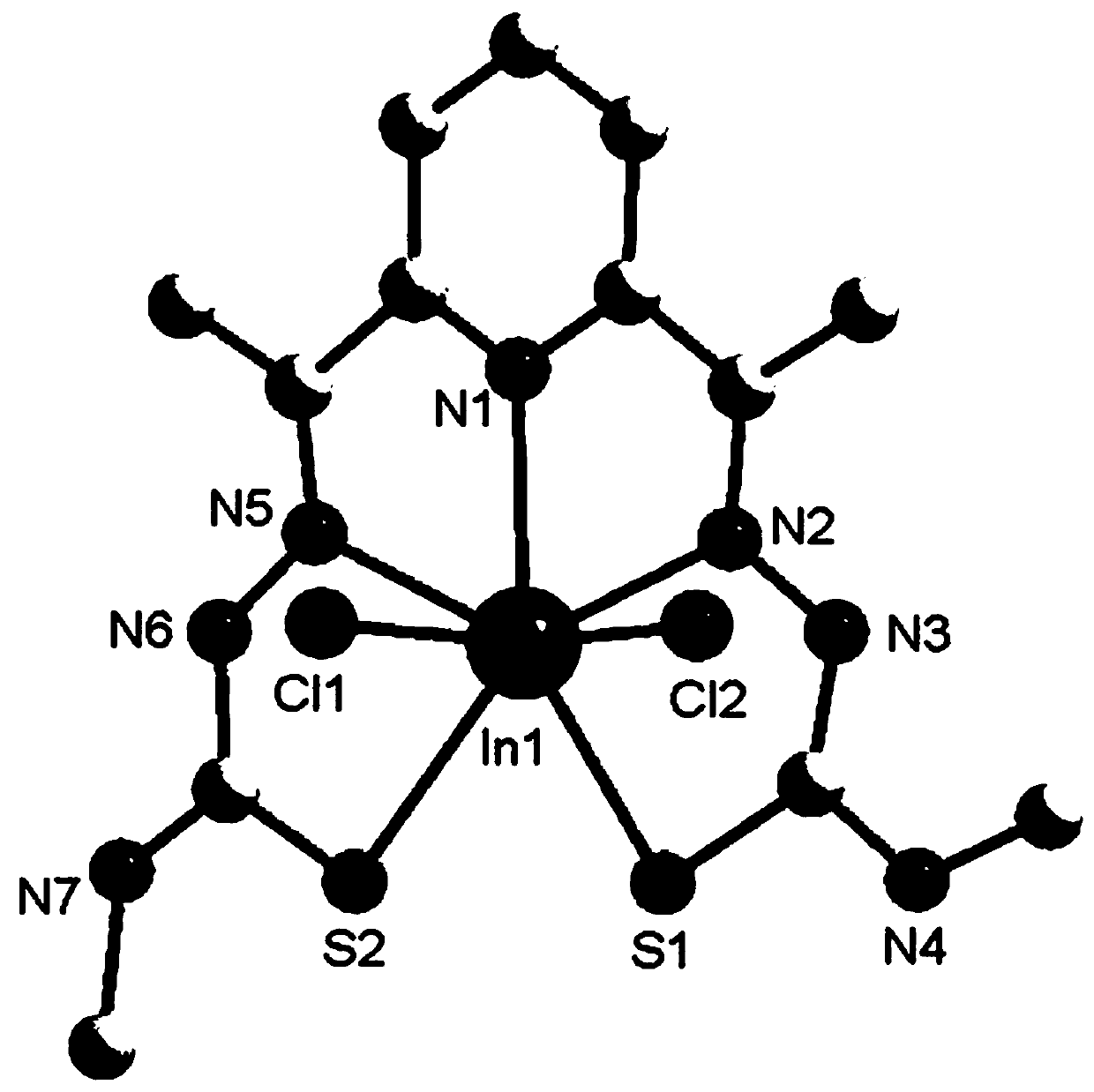
[0040] (3) Take InCl 3 (0.221g, 1mmol) was dissolved in 20mL of methanol solution, and then added dropwise to 10mL of ethanol solution containing 1mmol of 2,6-diacetylpyridine 4-methylthiosemicarbazide ligand, and stirred at 60°C for 6h under reflux, and After the reaction, the solution was filtered into a 50mL beaker, sealed with plastic wrap, needled into 6 holes, and volatilized at room temperature for several days to obtain light yellow crystal C2. The single cryst...
Embodiment 3
[0043] The synthesis of indium compound C3 comprises the following steps:
[0044] (1) Dissolve 2,6-diacetylpyridine (0.815g, 5mmol) in 20mL of ethanol and stir at 60°C for 15min to obtain a solution;
[0045](2) Add 4-phenylthiosemicarbazide (1.67g, 10mmol) to the prepared solution, reflux and stir at 60°C for 12h, after the reaction, cool to room temperature and pour it into a beaker for volatilization, and filter the obtained pale yellow precipitate , washed 3 times with absolute ethanol to obtain ligand L3;
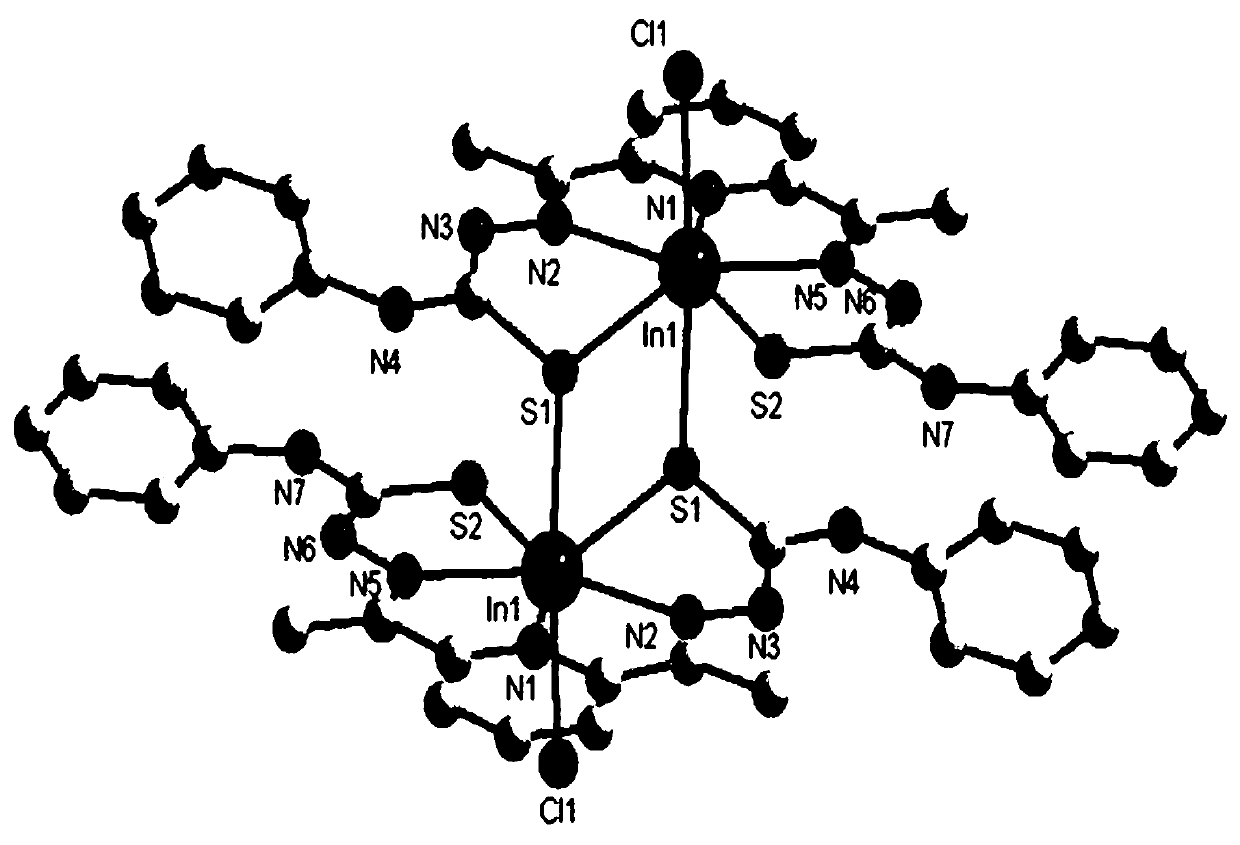
[0046] (3) Take InCl 3 (0.221g, 1mmol) was dissolved in 20mL of methanol solution, and then added dropwise to 10mL of ethanol solution containing 1mmol of 2,6-diacetylpyridine 4-phenylthiosemicarbazide ligand, and stirred at reflux at 60°C for 6h. After the reaction, the solution was filtered into a 50mL beaker, sealed with plastic wrap, needled into 6 holes, and volatilized at room temperature for several days to obtain yellow crystal C3 with a single crystal struc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com