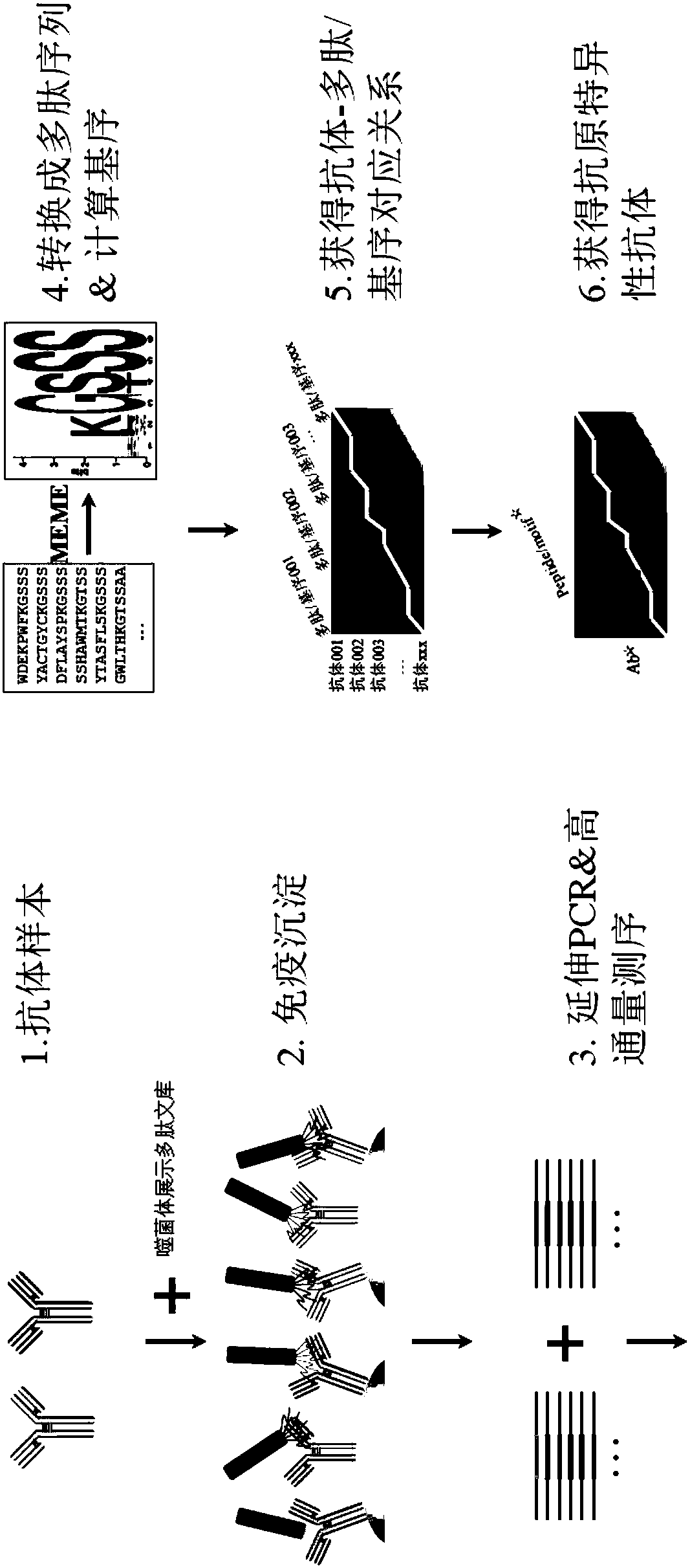
A method for rapidly preparing an antigen-specific antibody
A specific and antibody-based technology, applied in chemical instruments and methods, biochemical equipment and methods, specific peptides, etc., can solve the problems of rapid acquisition of antigen-specific antibodies that are not involved or involved, and achieve accelerated acquisition, accuracy and The effect of high reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Example 1. Establishing an antibody-polypeptide relationship table
[0069] 1. Purchase Ph.D. from NEB Company TM -12phage display peptide library kit (New England Biolabs Ltd., Cat. No. E8110S);
[0070] 2. At 4°C, use 3% BSA-containing TBST-II (TBS solution containing 0.5% Tween 20, see Table 1 for its components) solution to block the 96-well PCR plate overnight, 200 μL per well;
[0071] Table 1
[0072]
[0073] 3. Add 0.4 μg of antibody to each well of the PCR plate. As a control, no antibody was added to one of the wells;
[0074] 4. Add 10 μL phage random peptide display library (NEB TM Ph.D-12), 100 times the diversity of the library;
[0075] Incubate overnight at 5.4°C;
[0076] 6. Add 10 μL of protein G magnetic beads (Thermo Fisher Scientific Inc., Cat. No. 10003D). Immunoprecipitation for 4 hours at 4°C;
[0077] 7. Use a 96-well magnetic stand to separate the magnetic beads. Wash 3 times with 200 μL TBST-II solution;
[0078] 8. Wash once wi...
Embodiment 2
[0135] Example 2, establishing antibody-motif relationship
[0136] 1. According to the requirements of the program MEME for the input file, input the polypeptide sequence corresponding to each antibody in the antibody-polypeptide relationship, calculate and output the 8 motifs, motif matrix and corresponding E value corresponding to the antibody, among which the The sequence matrix represents the contribution degree of each amino acid residue in the given position of the corresponding motif; the calculation of the motif matrix adopts a well-known method MEME.
[0137] 2. With 0.05 as the threshold, select motifs with E values less than 0.05 and the corresponding motif matrix;
[0138] 3. Integrate all antibodies, E values and motif matrixes to establish antibody-motif relationship tables (see Table 7 and Table 8).
[0139] Table 7 Antibody-Motif Relationship Table 1
[0140]
[0141]
[0142]
[0143] Table 8 Correspondence between antigens and motifs Table 2 ...
Embodiment 3
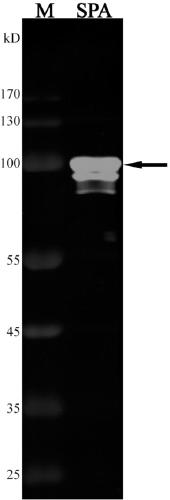
[0147] Example 3. Obtaining an antibody targeting protein SPA
[0148] 1. Using the polypeptide sequence in the antibody-polypeptide relationship table, the amino acid sequence SEQ ID No.33 corresponding to the antigen SPA (such as Figure 4 shown) comparison, it was found that a polypeptide ASWGSPHNRTSH recognized by the antibody Ab048 was completely identical to a part of the amino acid sequence in the antigen SPA, and the antibody Ab048 against the antigen SPA was obtained immediately;
[0149] 2. Use 10% separation gel, the loading volume is 10 μL, absorb 8 μL of protein SPA, and 2 μL of SDS-PAGE loading buffer, after mixing, denature at 95°C for 10 minutes. The sample loading volume was 10 μL, and 5 μL of molecular weight standards were added at the same time for SDS-PAGE electrophoresis. The electrophoresis conditions are 80V, 30min; 120V, 90min;
[0150] 3. Use cellulose acetate membrane. The membrane transfer condition is 160mA, 78min; (see Table 9 for the formulati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com