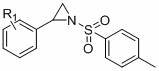
A kind of method for preparing 1,4-dihydrooxazine
A technology for dihydrooxazine and derivatives, which is applied in the field of preparation of 1,4-dihydrooxazine, can solve the problems of inapplicability to the preparation of multi-substituted oxazine rings, high preparation costs, narrowing of the substrate range, etc., and achieve suitable It is suitable for large-scale industrial production, reduces the preparation cost, and has the effect of less reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
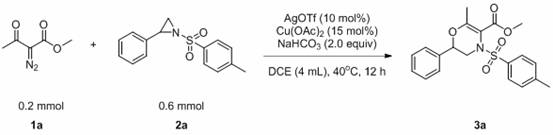
[0033] The reaction flask was filled with compound 1a (0.2 mmol, 28.4 mg), compound 2a (0.6 mmol, 163.8 mg), AgOTf (0.03 mmol, 7.7 mg), Cu(OAc) 2 (0.02 mmol, 3.6 mg), NaHCO 3 (0.4 mmol, 33.6 mg), DCE (0.4 mL). Then the system was heated in the air at 40°C for about 12 hours, the solvent was removed by a rotary evaporator, adsorbed on silica gel, and the product 3a was obtained by simple column chromatography with a yield of 70%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0034] 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.3 Hz, 2H), 7.35 (ddd, J = 8.3, 6.7, 4.6 Hz, 5H), 7.13 (dd, J = 7.5, 1.8 Hz, 2H) , 4.36 (dd, J = 10.2,2.9 Hz, 1H), 3.85 (s, 3H), 2.85 (dd, J = 14.6, 10.3 Hz, 1H), 2.45 (s, 3H), 2.26 (s, 3H); 13C NMR (101 MHz, CDCL3) Δ 166.12, 154.17, 144.66, 136.27,134.59, 129.92, 128.91, 128.28, 125.91, 106.08, 75.22, 52.06, 48.31.67,...
Embodiment 2
[0036]
[0037] The reaction flask was filled with compound 1b (7 mmol, 1.1 g), compound 2a (21 mmol, 1.9 g), AgOTf (0.7 mmol, 0.2 g), Cu(OAc) 2(1.1 mmol, 0.2 g), NaHCO 3 (14 mmol, 1.2 g), DCE (14 mL). Then the system was heated in the air at 40°C for about 12 hours, and the solvent was removed by a rotary evaporator, adsorbed on silica gel, and the product 3b was obtained by simple column chromatography with a yield of 73%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0038] 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 8.2 Hz, 2H), 7.48 – 7.26 (m,5H), 7.14 (dd, J = 7.5, 1.6 Hz, 2H), 4.40 (dd, J = 10.2, 2.8 Hz, 1H), 4.34(dt, J = 13.8, 6.9 Hz, 2H), 3.82 (dd, J = 14.6, 3.0 Hz, 1H), 2.84 (dd, J = 14.6, 10.3 Hz, 1H), 2.45 (s, 3H), 2.26 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H); 13CNMR (101 MHz, CDCl3) δ 165.70, 153.81, 144.58, 136.37, 134.65, 129.86, 128.87, 12...
Embodiment 3
[0040]
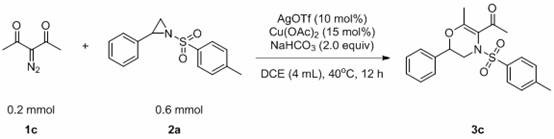
[0041] The reaction flask was filled with compound 1c (0.2 mmol, 25.2 mg), compound 2a (0.6 mmol, 163.9 mg), AgOTf (0.03 mmol, 7.7 mg), Cu(OAc) 2 (0.02 mmol, 3.6 mg), NaHCO 3 (0.4 mmol, 33.6 mg), DCE (0.4 mL). Then the system was heated at 40°C in the air for about 12 hours, and the solvent was removed by a rotary evaporator, adsorbed on silica gel, and the product 3a was obtained by simple column chromatography with a yield of 80%. The main test data of the obtained product are as follows. It can be seen from the analysis that the actual synthesized product is consistent with the theoretical analysis.
[0042] 1 H NMR (400 MHz, CDCl 3 ) δ 7.72 (d, J = 8.3 Hz, 2H), 7.42 (d, J = 8.0Hz, 2H), 7.36 – 7.31 (m, 3H), 7.04 – 6.98 (m, 2H), 4.03 (dd, J = 14.9, 3.1Hz, 1H), 3.72 (dd, J = 10.6, 3.1 Hz, 1H), 2.96 (dd, J = 14.9, 10.6 Hz, 1H), 2.48 (s, 3H), 2.47 (s, 3H), 2.14 (s, 3H); 13 C NMR (101 MHz, CDCl 3 ) δ 197.15,152.15, 144.63, 135.52, 133.03, 129.75, 128....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com