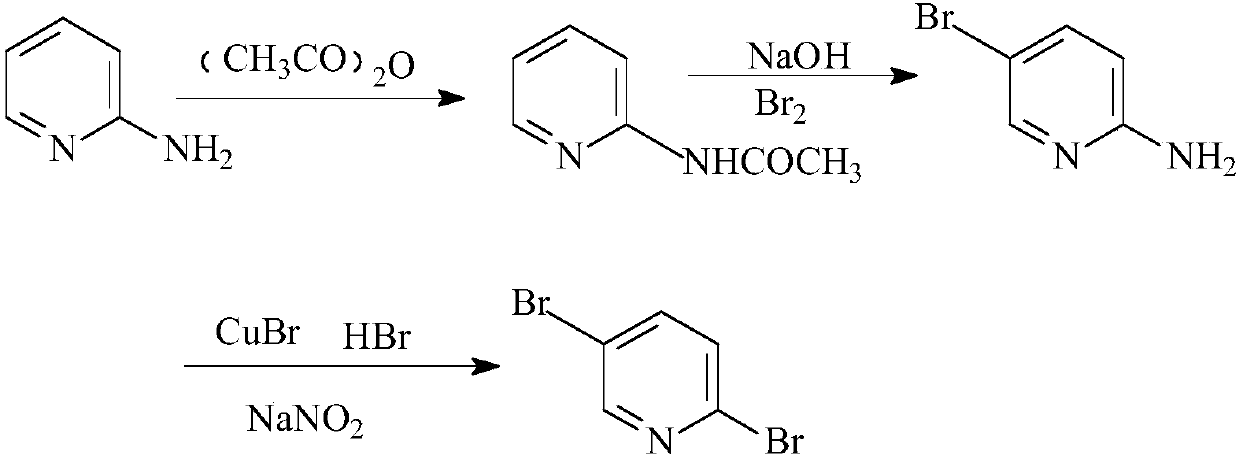
Synthesis method of 2, 5-dibromopyridine
A synthetic method, the technology of dibromopyridine, applied in the direction of organic chemistry, etc., can solve the problems of low yield and long process route, and achieve the effect of high yield, short process route and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 18.82g (0.2mol) of 2-aminopyridine and 30.63g (0.3mol) of acetic anhydride to a 200ml four-port oil bath and raise the temperature to reflux, follow the reaction with thin-layer chromatography until the reaction is complete, and wait until the temperature of the reaction solution drops to 20-25 At ℃, slowly add 35.2g (0.22mol) of liquid bromine dropwise, and react at 50℃ for 2.5 hours after dropping. Add water to the system until the solid is completely dissolved, slowly add 80ml of 40% sodium hydroxide solution dropwise, a large amount of solid is formed, continue the reaction for 30 minutes, filter with suction, dry, and recrystallize with ethanol to obtain 2-amino-5-bromopyridine 14.1 g, yield 65% (molar yield).
[0022] Add 50ml48% hydrobromic acid solution in the 200ml there-necked flask equipped with stirrer and thermometer, 6.9g (0.048mol) cuprous bromide is dissolved in the hydrobromic acid solution, keep temperature 0 ℃ in ice-water bath and slowly add 6g (...
Embodiment 2
[0024] Add 18.82g (0.2mol) of 2-aminopyridine and 20.4g (0.2mol) of acetic anhydride to a 200ml four-port oil bath and raise the temperature to reflux, follow the reaction with thin-layer chromatography until the reaction is complete, and wait until the temperature of the reaction solution drops to 20-25 At ℃, slowly add 35.2g (0.22mol) of liquid bromine dropwise, and react at 55℃ for 2 hours after dropping. Add water to the system until the solid is completely dissolved, slowly add 80ml of 40% sodium hydroxide solution dropwise, a large amount of solid is formed, continue the reaction for 30 minutes, filter with suction, dry, and recrystallize with ethanol to obtain 2-amino-5-bromopyridine 12.98 g, yield 60% (molar yield).
[0025] Add 50ml of 48% hydrobromic acid solution to a 200ml three-necked flask equipped with a stirrer and a thermometer, dissolve 7.5g (0.052mol) of cuprous bromide in the hydrobromic acid solution, and slowly add 6g in an ice-water bath to maintain the ...
Embodiment 3
[0027] Add 18.82g (0.2mol) of 2-aminopyridine and 40.8g (0.4mol) of acetic anhydride to a 200ml four-port oil bath and raise the temperature to reflux, follow the reaction with thin-layer chromatography until the reaction is complete, and wait until the temperature of the reaction solution drops to 20-25 At ℃, slowly add 35.2g (0.22mol) of liquid bromine dropwise, and react at 45℃ for 3 hours after dropping. Add water to the system until the solid is completely dissolved, slowly add 80ml of 40% sodium hydroxide solution dropwise, a large amount of solid is formed, continue the reaction for 30 minutes, filter with suction, dry, and recrystallize with ethanol to obtain 2-amino-5-bromopyridine 12.5 g, yield 62% (molar yield).
[0028] Add 50ml48% hydrobromic acid solution in the 200ml there-necked flask equipped with agitator and thermometer, 8.6g (0.06mol) cuprous bromide is dissolved in the hydrobromic acid solution, and slowly add 6g ( 0.04mol) 2-amino-5-bromopyridine, keep t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com