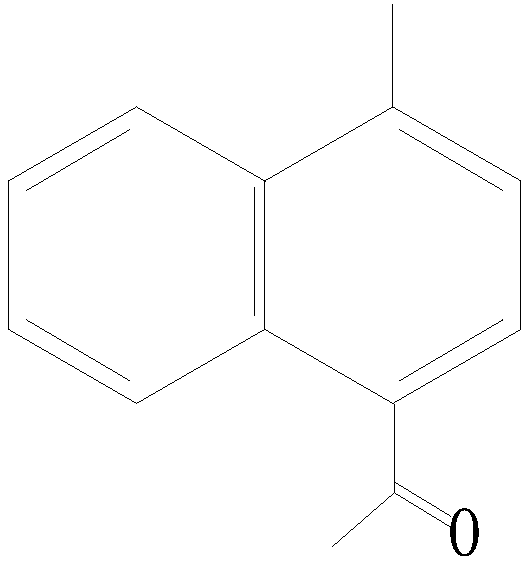
Method for purifying 4-acetyl-1-methyl naphthalene in acetyl methyl naphthalene mixture
A technology of acetyl-methylnaphthalene mixture and acetyl group, which is applied in the field of purifying 4-acetyl-1-methylnaphthalene, can solve the problems of high requirements for rectification equipment, large production investment cost, and large environmental impact, and achieve improvement The effect of purity and yield, simple method and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] A method for purifying 4-acetyl-1-methylnaphthalene from an acetylmethylnaphthalene mixture. The acetylmethylnaphthalene mixture is placed in a melting crystallization device, the temperature is lowered from normal temperature to 12°C, and the uncrystallized mother liquor is released , and then the crystallized solid starts to heat up and sweat from 12°C, the sweating temperature is 15-37°C, and the average heating rate is 2.5°C / h. Stop sweating until the sweat product content reaches 99.0%. Finally, the sweat is discharged, and the crystalline solid in the melting and crystallization device is heated and melted to obtain 4-acetyl-1-methylnaphthalene.
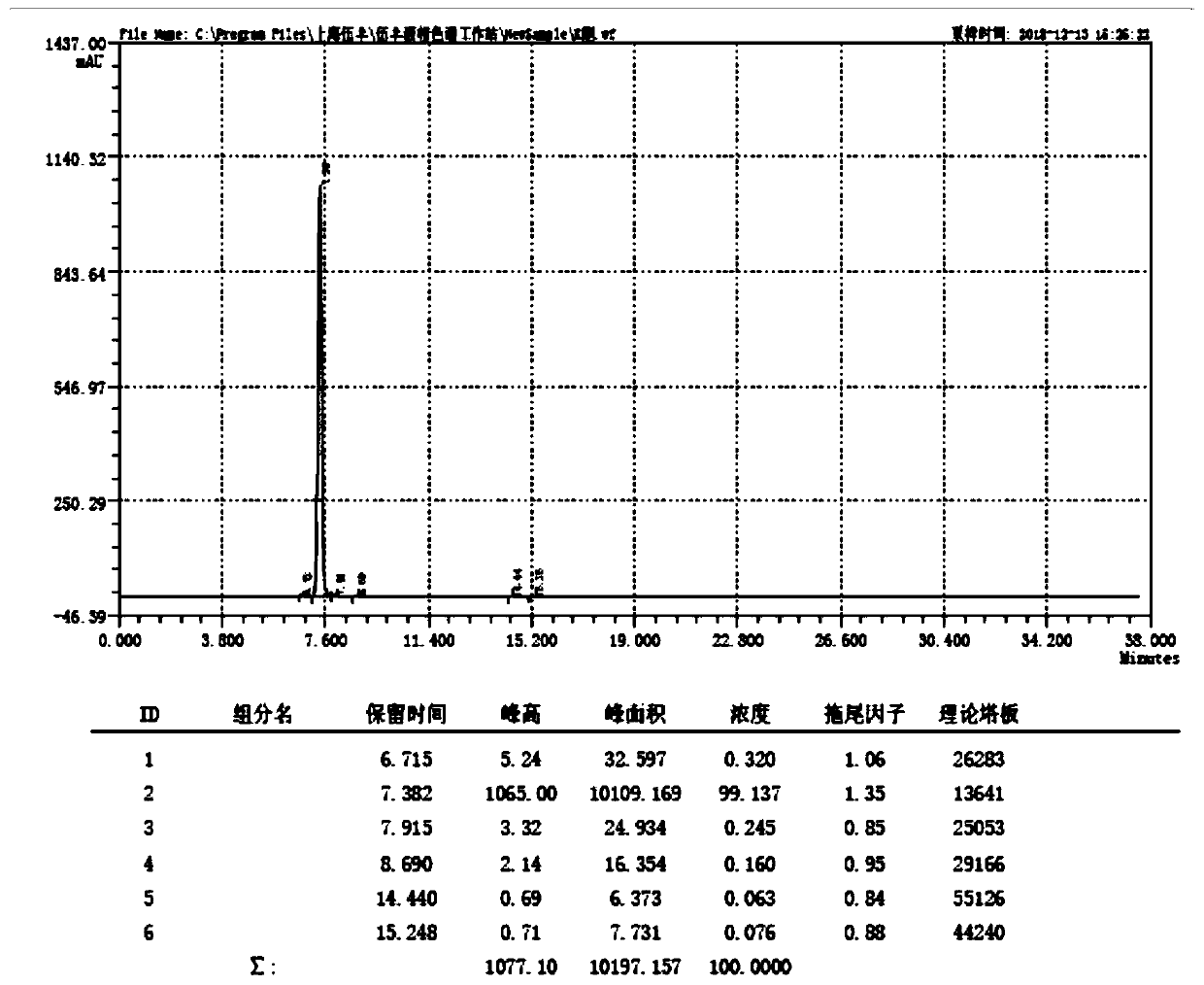
[0016] The purity of the 4-acetyl-1-methylnaphthalene obtained in this example is 99.2%.
Embodiment 2
[0018] A method for purifying 4-acetyl-1-methylnaphthalene from an acetylmethylnaphthalene mixture. The acetylmethylnaphthalene mixture is placed in a melting crystallization device, the temperature is lowered from normal temperature to 12°C, and the uncrystallized mother liquor is released , and then the crystallized solid starts to heat up and sweat from 12°C, the sweating temperature is 15-37°C, and the average heating rate is 1.5°C / h. Stop sweating until the sweat product content reaches 99.0%. Finally, the sweat is discharged, and the crystalline solid in the melting and crystallization device is heated and melted to obtain 4-acetyl-1-methylnaphthalene.
[0019] The purity of the 4-acetyl-1-methylnaphthalene obtained in this example is 99.4%.
Embodiment 3
[0021] A method for purifying 4-acetyl-1-methylnaphthalene from an acetylmethylnaphthalene mixture. The acetylmethylnaphthalene mixture is placed in a melting crystallization device, the temperature is lowered from normal temperature to 12°C, and the uncrystallized mother liquor is released , and then the crystallized solid starts to heat up and sweat from 12°C, the sweating temperature is 15-37°C, and the average heating rate is 0.5°C / h. Stop sweating until the sweat product content reaches 99.0%. Finally, the sweat is discharged, and the crystalline solid in the melting and crystallization device is heated and melted to obtain 4-acetyl-1-methylnaphthalene.
[0022] The purity of the 4-acetyl-1-methylnaphthalene obtained in this example is 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com