Test paper applicable to quantitative detection of peripheral blood immunochromatography and application of test paper
A technology of immunochromatography and quantitative detection, which is applied in the field of medical detection, can solve problems such as difficulty in accurate detection and interference of red blood cell detection, and achieves the effects of easy production, wide linear detection range, and easy promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] In order to describe the technical content, achieved goals and effects of the present invention in detail, the following descriptions will be made in conjunction with the embodiments and accompanying drawings.
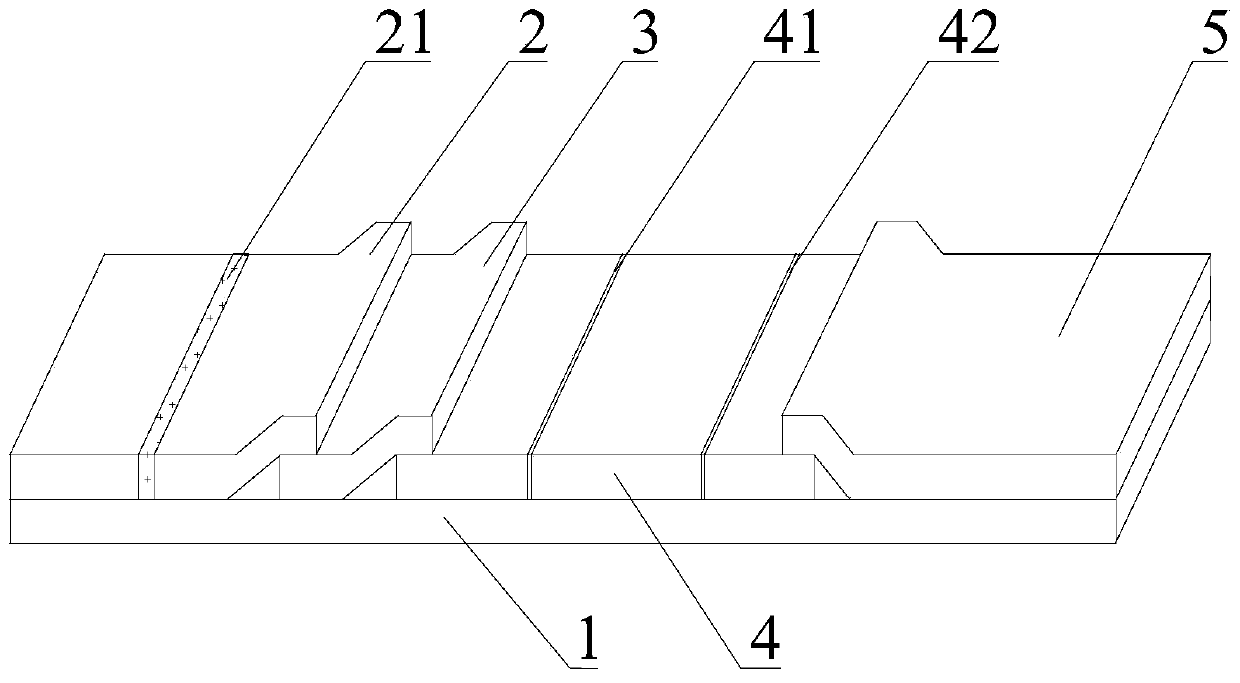
[0029] Embodiment 1 of the present invention is: a test paper suitable for quantitative detection of peripheral blood immune chromatography, such as figure 1 As shown, it includes a base plate 1 and an upper cover (not shown in the figure), the upper cover is covered on the base plate 1, and the base plate 1 is sequentially provided with a sample pad 2, a sealing pad 3, Reaction pad 4 and water-absorbing pad 5; The sample pad 2 is coated with a first antibody, the first antibody has a tracer marker and can recognize an antigenic epitope of the target to be tested; the blocking pad 3 RBC is coated on it, and the sealing pad 3 and the reaction pad 4 are partially laminated. The upper cover is provided with a sample injection hole and an observation window in the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com