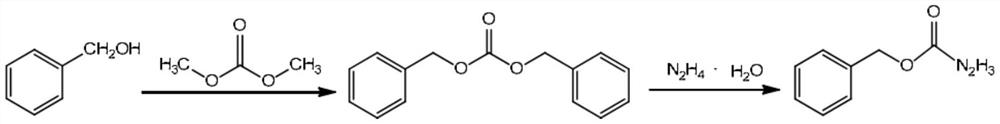
A kind of hydrazine hydrate treatment method after synthesis of benzyl carbazate
A technology of benzyl carbazate and a treatment method is applied in the field of hydrazine hydrate treatment after the synthesis of benzyl carbazate, which can solve problems such as reducing environmental protection pressure, and achieve the effects of reducing environmental protection pressure, controllable degradation, and improving activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Transfer 376g of benzyl carbazate synthesis reaction solution into a 500ml reaction bottle, cool down the system, when the temperature is ≤60°C, stop cooling, start negative pressure distillation, slowly heat up, control the vacuum of the system to 0.07-0.09MPa, and distill The end point temperature is 90°C, no distillate is separated out, and 63.5g fraction Ⅰ is obtained
[0021] (2) After Fraction I was collected, the temperature of the system continued to rise, the vacuum degree was controlled to be ≥ 0.095 MPa, the end point temperature of distillation was 120°C, no distillate was separated out, and 11.6 g of Fraction II was obtained.
[0022] (3) Add 1% Raney nickel catalyst into the reaction flask for fraction I, and react at a controlled temperature of 30° C. for 6 hours.
[0023] (4) After the reaction finishes, filter and collect the catalyst, and the catalyst can be recycled.
[0024] (5) Fraction II was added together with 1.7 g of alkali additive potass...
Embodiment 1
[0027] The reaction solution that the fraction II of Example 1 participates in the synthesis of benzyl carbazate is processed:
[0028] (1) Transfer 381g of benzyl carbazate synthesis reaction solution into a 500ml reaction bottle, cool down the system, when the temperature is ≤60°C, stop the cooling, start negative pressure distillation, slowly raise the temperature, control the vacuum degree of the system to 0.07-0.09MPa, and distill The end point temperature was 90°C, no distillate was separated out, and 57.2 g of fraction I were obtained.
[0029] (2) After Fraction I was collected, continue to heat up the system, control the vacuum degree ≥ 0.095MPa, and the distillation end point temperature was 120°C. No distillate was separated out, and 13.4g of Fraction II was obtained.
[0030] (3) Fraction I was added to the reaction flask with the recovered Raney nickel catalyst filtered after the reaction in Example 1, and the reaction was carried out at a controlled temperature o...
Embodiment 2
[0035] The reaction solution that the fraction II of embodiment 2 participates in the synthesis of benzyl carbazate is processed:
[0036] (1) Transfer 379g of synthetic benzyl carbazate reaction solution into a 500ml reaction bottle, cool down the system, when the temperature is ≤60°C, stop cooling, start negative pressure distillation, slowly heat up, control the vacuum degree of the system to be 0.07-0.09MPa, and distill The end point temperature is 90°C, no distillate is separated out, and 67.2g fraction I is obtained
[0037] (2) After Fraction I was collected, the temperature of the system continued to rise, the vacuum degree was controlled to be ≥0.095 MPa, the end point temperature of the distillation was 120°C, no distillate was detected, and 12.8 g of Fraction II was obtained.
[0038] (3) Fraction I was added to the reaction flask with the recycled Raney nickel catalyst filtered after the reaction in Example 2, and the reaction was carried out at a controlled temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com