miRNA detection fluorescent biological probe and detection method and application thereof
A fluorescent biological probe and fluorescence technology, which is applied in the field of biosensors, can solve the problem that miRNA detection methods cannot be fast, simple and low-cost at the same time, and achieve the effects of label-free, high specificity and simple steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
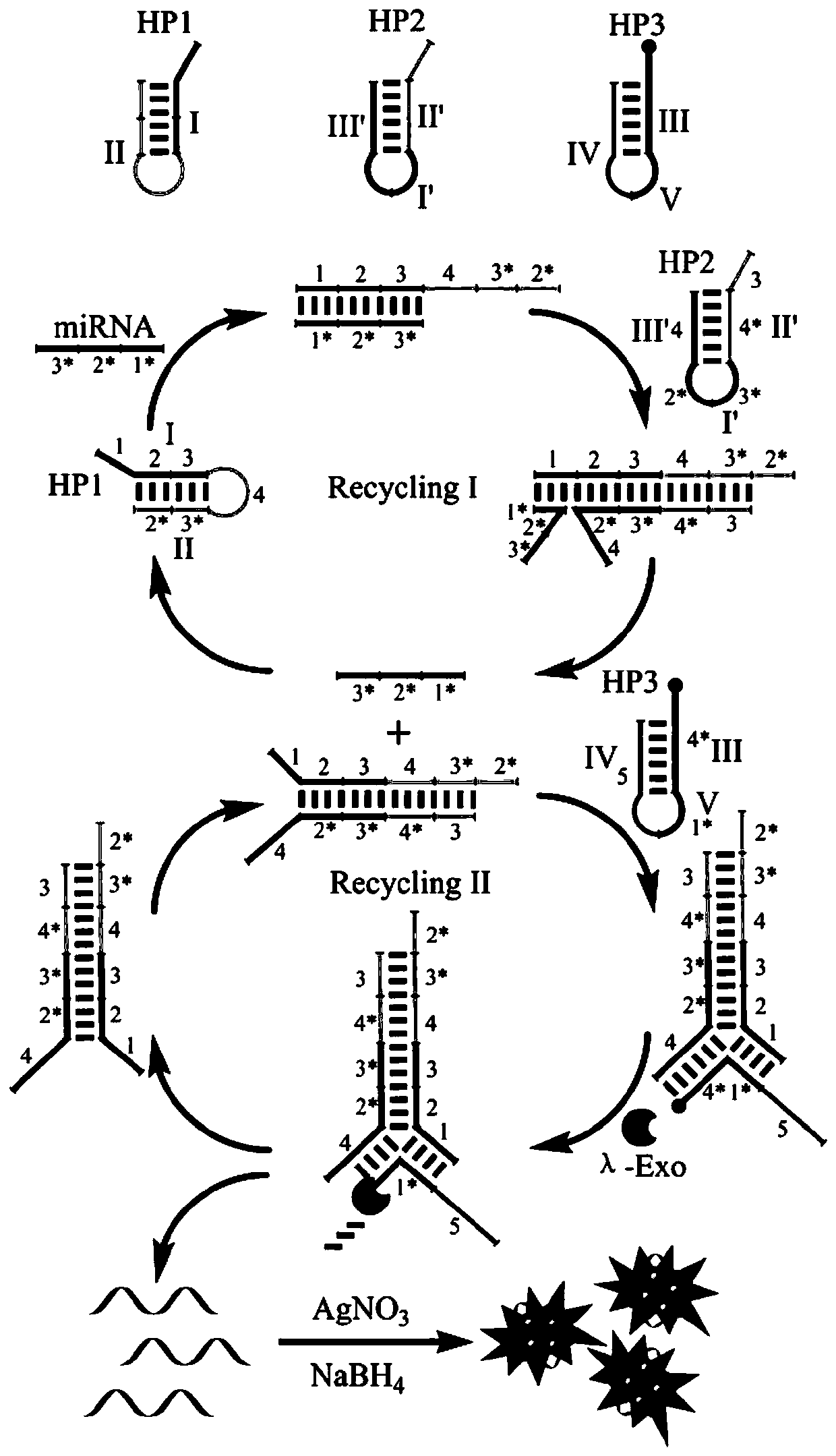
[0077] Embodiment 1 detects the design of the fluorescent biological probe of miRNA
[0078] The fluorescent bioprobe for detecting miRNA in this embodiment includes a first hairpin, a second hairpin and a third hairpin; the first hairpin, the second hairpin and the third hairpin are all single-stranded linear molecules After self-folding, the complementary bases in the folding region are hybridized, and the part of the local region that forms a double-stranded structure is the stem region, and the part that does not form a double-stranded structure and folds back is the loop region;
[0079] The first hairpin includes: domain (I), which can hybridize complementary to the target miRNA; domain (II), which partially hybridizes with the domain (I) to form a hairpin structure;
[0080] The second hairpin includes: a structural domain (I'), which can partially hybridize with the structural domain (I) of the first hairpin; a structural domain (II'), which is connected to one end of ...
Embodiment 2
[0095] Embodiment 2 detects the fluorescent biosensor of miRNA
[0096] This embodiment provides a fluorescent biosensor for detecting miRNA, including an A reaction system and a B reaction system;
[0097] The A reaction system, in terms of 50 μL, includes:
[0098] miRNA to be tested, 4 μL;
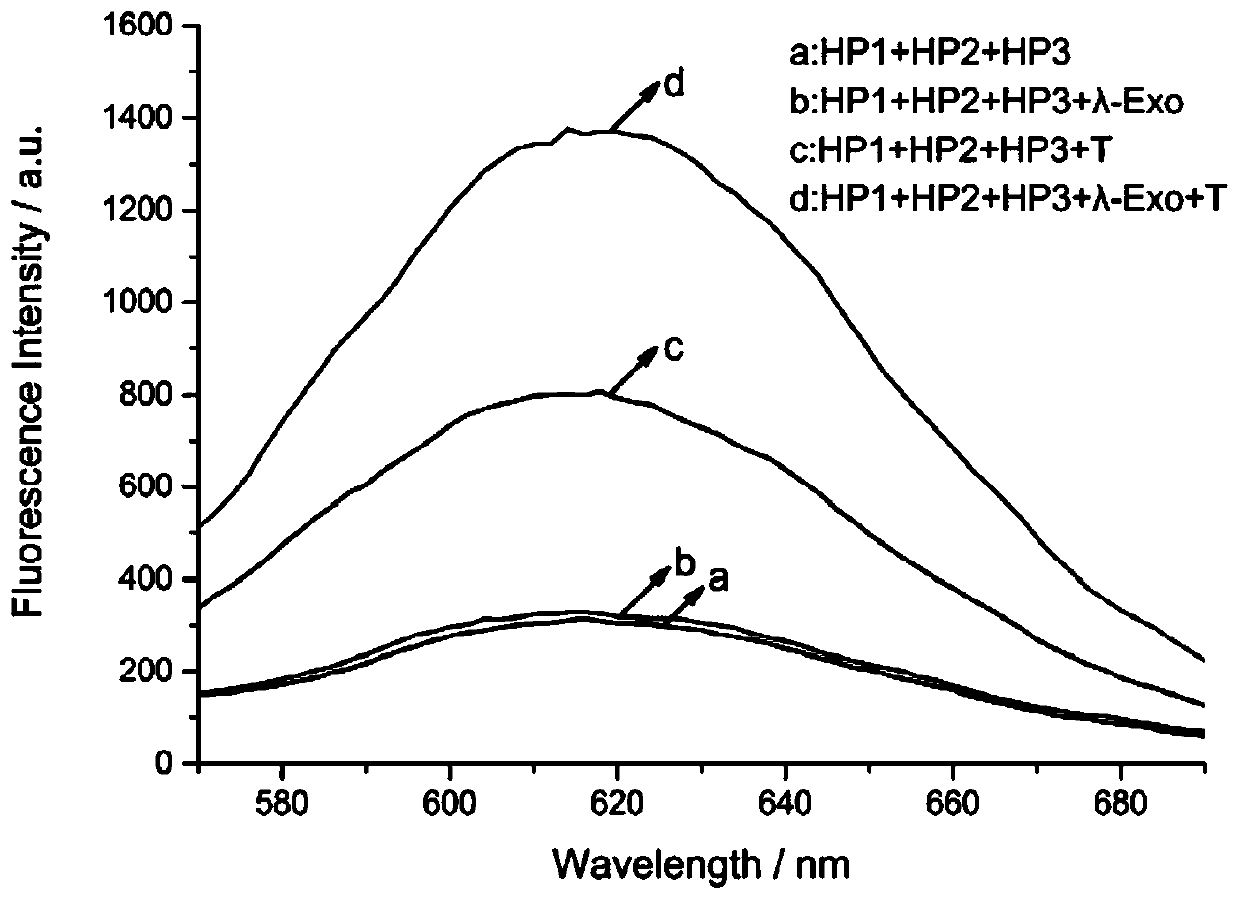
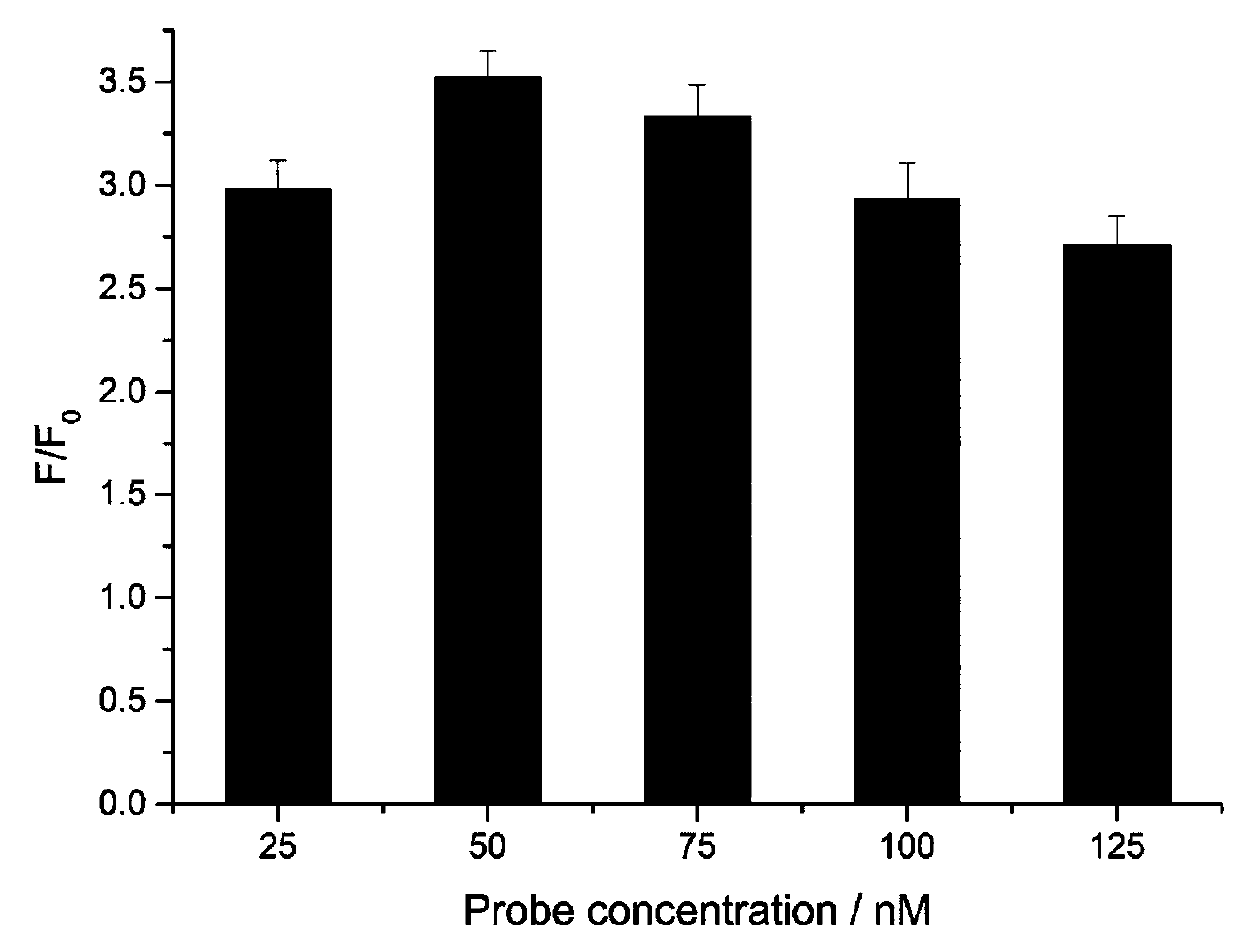
[0099] Probe solution, containing hairpin HP1, hairpin HP2 and hairpin HP3 in a molar ratio of 1:1:1, the concentration of hairpin HP1 is 50 nM (nmol / L), 17 μL;
[0100] The first buffer solution contains Tris-HNO with a concentration of 15mM 3 , a concentration of 55mM KNO 3 , a concentration of 5 mM Mg(NO 3 ) 2 , and DTT (dithiothreitol) at a concentration of 1.5 mM, pH 7.8, 18 μL;
[0101] The RNase inhibitor, 15U, 6 μL;
[0102] The exonuclease λ-Exo, 15U, 4 μL;
[0103] wxya 2 O make up.
[0104] The B reaction system, in terms of 50 μL, includes:
[0105] The second buffer containing Ag ions is AgNO 3 A sodium citrate solution in which AgNO 3 The molar ratio with the ...
Embodiment 3
[0108] Embodiment 3 detects the fluorescent biosensor of miRNA
[0109] This embodiment provides a fluorescent biosensor for detecting miRNA, including an A reaction system and a B reaction system;
[0110] The A reaction system, in terms of 50 μL, includes:
[0111] miRNA to be tested, 6 μL;
[0112] Probe solution, containing hairpin HP1, hairpin HP2 and hairpin HP3 in a molar ratio of 1:1:3, the concentration of hairpin HP1 is 50 nM (M is mol / L), 13 μL;
[0113] The first buffer solution contains Tris-HNO with a concentration of 25mM 3 , a concentration of 45mM KNO 3 , a concentration of 15 mM Mg(NO 3 ) 2 , and DTT (dithiothreitol) at a concentration of 0.5 mM, pH 8.0, 22 μL;
[0114] The RNase inhibitor, 5U, 4 μL;
[0115] The exonuclease λ-Exo, 25U, 6 μL;
[0116] Top up with deionized water.
[0117] The B reaction system, in terms of 50 μL, includes:
[0118] The second buffer containing Ag ions is AgNO 3 A sodium citrate solution in which AgNO 3 The molar r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com