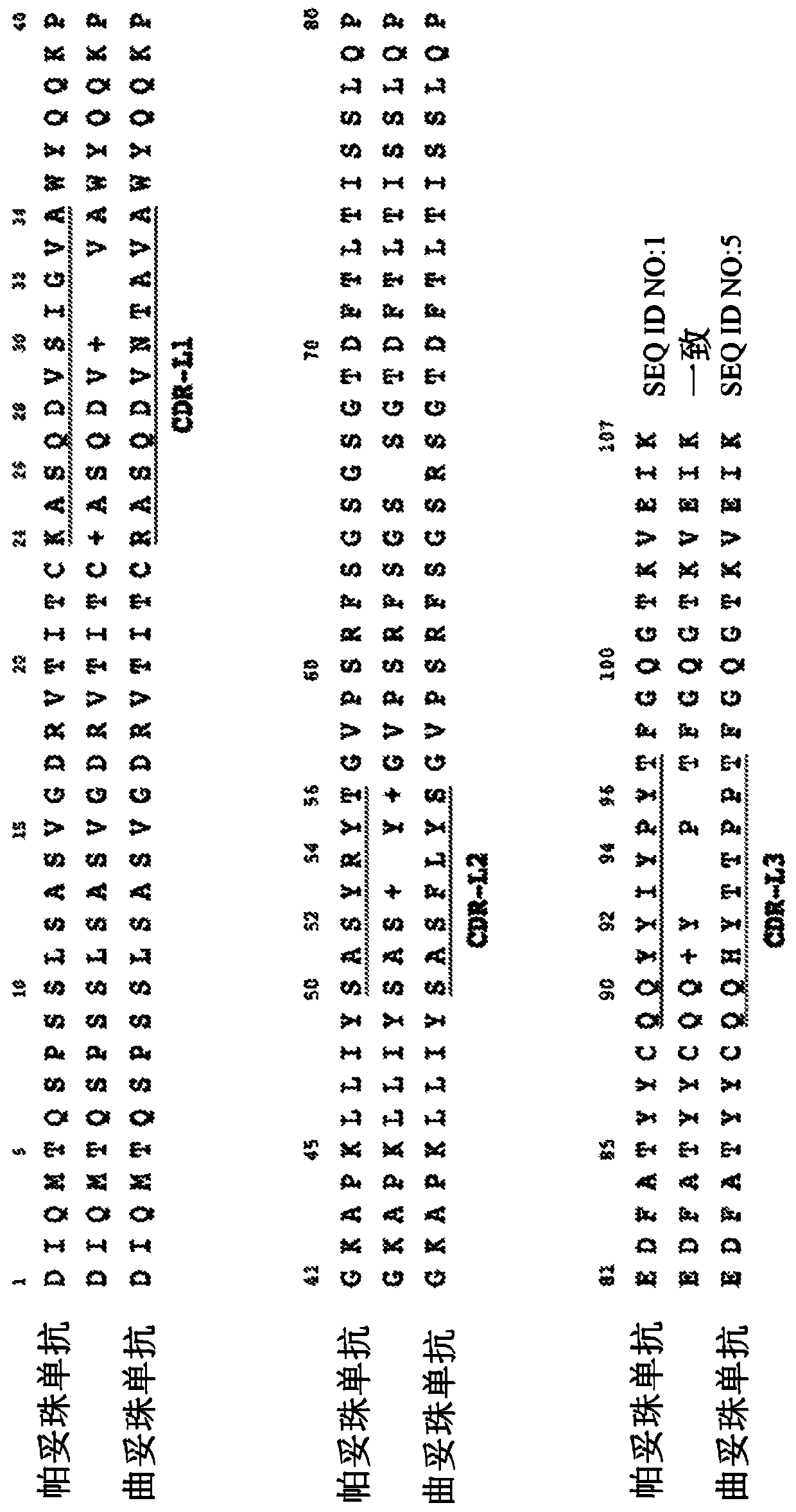
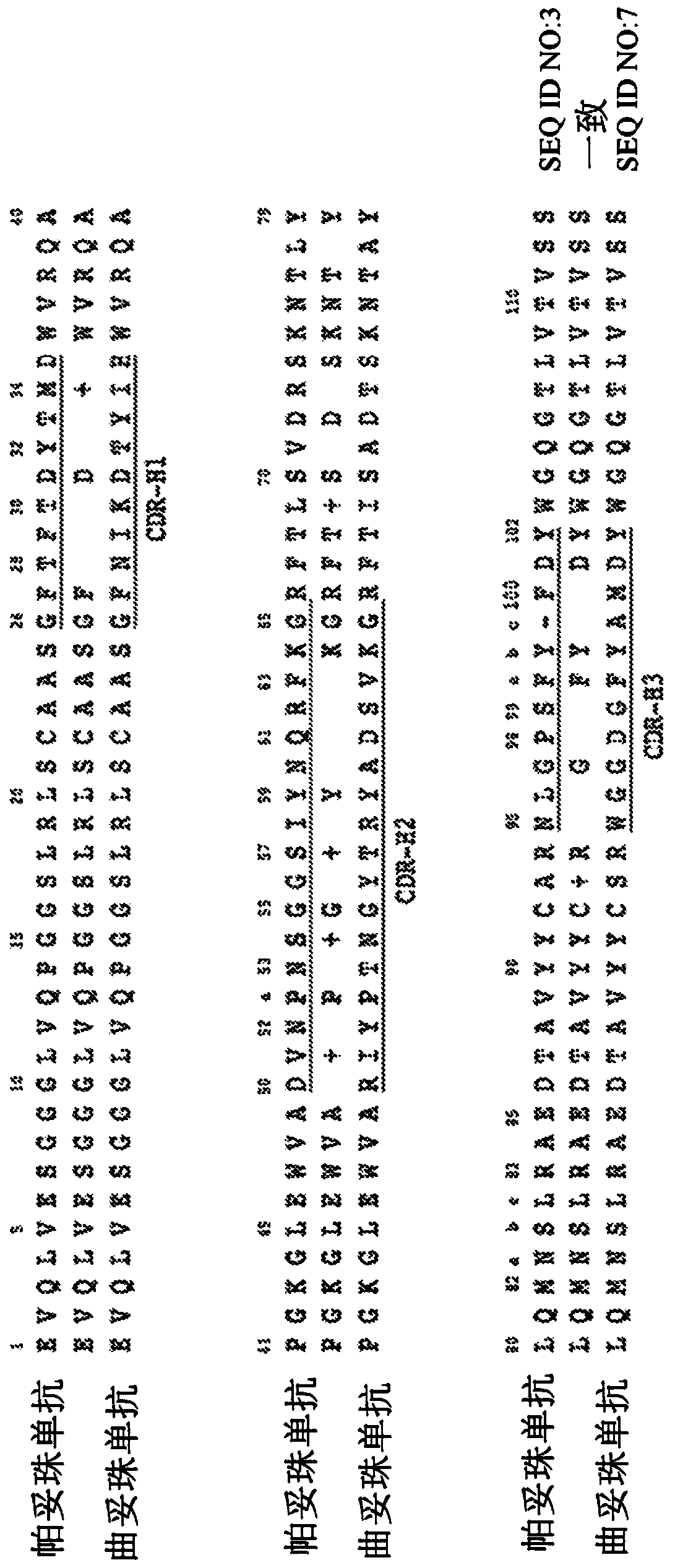
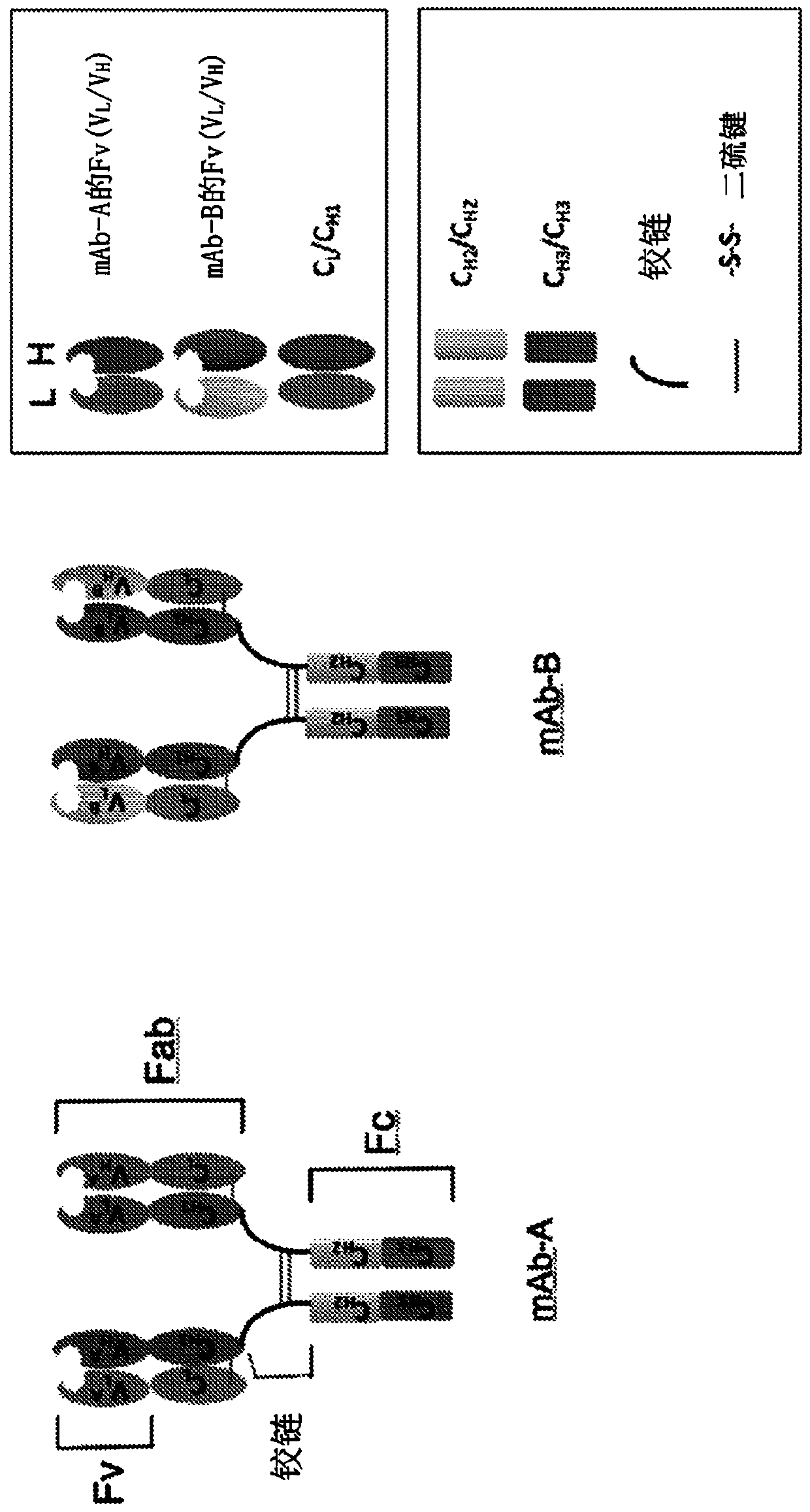
Biparatopic and multiparatopic antibodies with common light chain and method of use
A paratope, light chain technology, applied in the field of diagnosis and treatment of human diseases, can solve the problem of not being able to maintain the binding affinity of two paratopes at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Example 1. Binding Potency of Antibodies Comprising Pertuzumab or Trastuzumab Heavy and Common Light Chains.
[0151] The binding potency of the antibody to the antigen HER2 was determined using an ELISA assay. The HER2 extracellular domain was coated on a 96-well plate overnight by adding 0.2ug / 100ul / well of the HER2 extracellular domain solution. Plates were washed 6 times with PBS, blocked with 3% BSA, and incubated for 2 hours. Plates were again washed 6 times with PBS. Anti-HER2 antibody was then added to each well at various concentrations indicated in the figure. After 1 hour of binding, the plate was washed 6 times with PBS. Horseradish peroxidase (HRP)-conjugated secondary antibodies were added and incubated for 1 hr. Add HRP substrate TMB to each well and read luminescence on a plate reader. The data were then fitted to a 4 parameter sigmoid curve to generate IC50s. Each sample was performed in triplicate.
[0152] Figure 8A Results are shown for va...
Embodiment 2
[0153] Example 2. Antibodies comprising modified Pertuzumab or Trastuzumab heavy chains and corresponding wild-type light chains Body binding effect.
[0154] The binding potency of mAbs formed by pairing the modified heavy chain with its wild-type light chain was determined using the same ELISA assay described above (see Table 4). mAbs formed with all three modified Pertuzumab heavy chains showed improved performance compared to Pertuzumab (mAb T1 ) (Fig. 9A). Compared with trastuzumab (mAb T22), mAbs formed with all modified trastuzumab heavy chains showed broadly similar performance, with four out of six showing moderate improvements. (FIG. 9B).
Embodiment 3
[0155] Example 3. Comprising Antibodies of Modified Pertuzumab or Trastuzumab Heavy Chains and Various Common Light Chains Combine potency.
[0156] The binding potency of mAbs formed by pairing the modified heavy chain with various common light chains was determined using the same ELISA assay described above (see Table 5). For mAbs using a modified Pertuzumab heavy chain, the best performer in this experiment was the inclusion of both the T30A and G56A substitutions in the heavy chain, which were comparable to the inclusion of N30S, mAb T43 paired with a common light chain replaced by S56Y and T94W. This light chain performed well in other pairings (consider T44 and T45; Figure 10). Performance was about the same for mAbs using the modified Trastuzumab heavy chain with N54T and D98T substitutions. mAb T47, which lacked the S56Y substitution in the common light chain, performed the worst, while mAb T46, which contained the N30S, S56Y and T94W substitutions in the common ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com