Copolymer composite micelles based on dynamic imine bond and preparation method thereof
A technology of composite micelles and imine bonds, which is applied in the field of preparation of copolymer composite micelles, can solve the problems of difficult research and development of new micelles, limited response sensitivity, instability, etc., and achieve good biocompatibility Good effect with biodegradability and biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1 Preparation of copolymer complex micelles (4:6) based on dynamic imine bonds
[0055] (1) Synthesis of terminal polymers
[0056] 1. Synthesis of aldehyde-terminated polyethylene glycol monomethyl ether (MPEG-CHO)
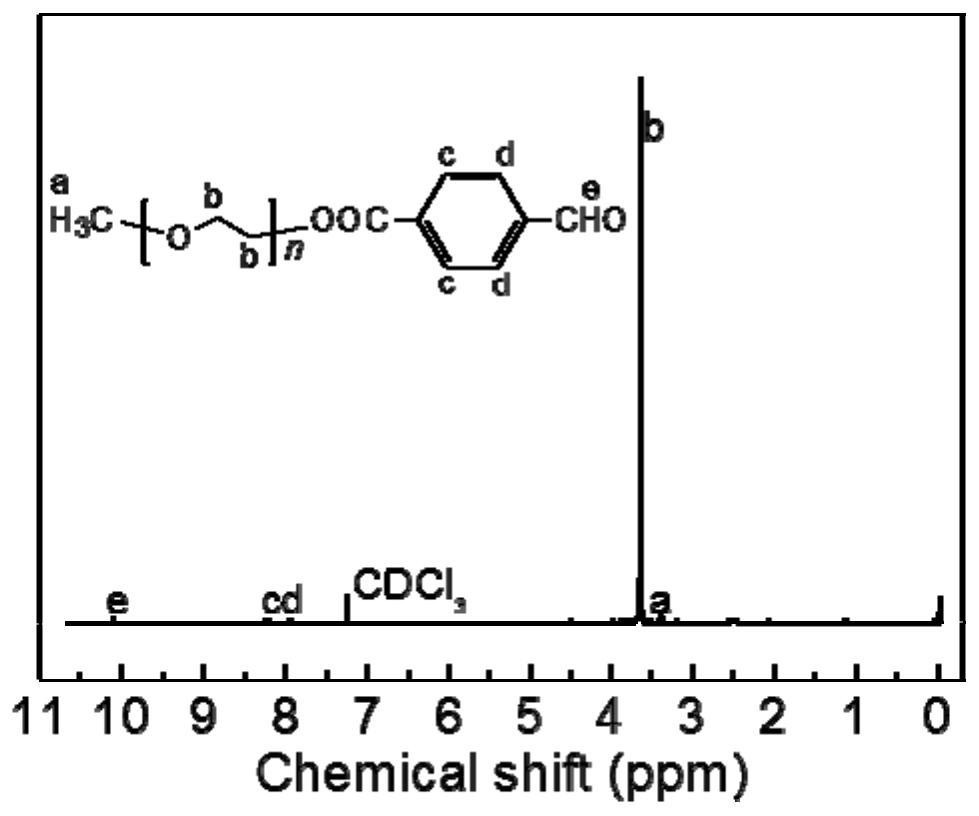
[0057] Weigh 10g (2mmol) MPEG and place it in a 250mL reaction flask, dissolve it with 100mL dichloromethane, then add 3g (20mmol) p-CBA, 4.1g (20mmol) DCC and 0.6g (5.0mmol) DMAP, and stir magnetically at 25°C Reaction 24h. After the reaction was completed, the reaction mixture was filtered, and the filtrate was concentrated by rotary evaporation, and the obtained solid was dissolved in 80 mL of isopropanol, and then left overnight at 4°C. After filtering, the resulting solid was washed with isopropanol and diethyl ether in turn, and vacuum-dried to obtain MPEG-CHO with a yield of 78%, which was set aside for later use. The detection and analysis results are as figure 1 shown.
[0058] figure 1 is MPEG-CHO 1 H NMR spectrum. figure 1 The...
Embodiment 2
[0098] Embodiment 2 Preparation of copolymer complex micelles (5:5) based on dynamic imine bonds
[0099] Weigh 12.75mg (0.00125mmol) of MPEG-b-PCL and 12.25mg (0.00125mmol) of PNVCL-b-PCL and dissolve in 5mL of tetrahydrofuran, stir at room temperature for 24h, then slowly drop 1.0mL of the above solution into ultrapure water, Until the aqueous solution turns into a light blue milky white solution, then set the volume to 10mL, dialyze for 48h to remove tetrahydrofuran, and obtain MPEG-b-PCL and PNVCL-b-PCL with a concentration of 0.5mg / mL and a molar ratio of 5:5. Copolymer Complex Micellar Solution. The detection and analysis results are as Figure 14 ~ Figure 15 shown.
[0100] Figure 14 It is a transmission electron micrograph of a copolymer composite micelle (5:5). From Figure 14 It can be seen that the micelles are uniformly distributed spherically.
[0101] Figure 15 is the particle size distribution diagram of the copolymer composite micelles (5:5). From F...
Embodiment 3
[0102] Embodiment 3 Preparation of copolymer composite micelles (6:4) based on dynamic imine bonds
[0103] Weigh 15.20mg (0.0015mmol) of MPEG-b-PCL and 9.80mg (0.0010mmol) of PNVCL-b-PCL and dissolve in 5mL of tetrahydrofuran, stir at room temperature for 24h, then slowly drop 1.0mL of the above solution into ultrapure water, Until the aqueous solution turns into a light blue milky white solution, then set the volume to 10mL, dialyze for 48h to remove tetrahydrofuran, and obtain MPEG-b-PCL and PNVCL-b-PCL with a concentration of 0.5mg / mL and a molar ratio of 6:4. Copolymer Complex Micellar Solution. The detection and analysis results are as Figure 16 ~ Figure 17 shown.
[0104] Figure 16 It is a transmission electron micrograph of a copolymer composite micelle (6:4). From Figure 16 It can be seen that the micelles are uniformly distributed spherically.
[0105] Figure 17 is the particle size distribution diagram of the copolymer composite micelles (6:4). From Fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com