Pharmaceutical formulations of suvorexant
A preparation and drug technology, applied in the field of stable pharmaceutical preparations, can solve the problems of unpredictable, unpredictable onset, unpredictable plasma concentration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
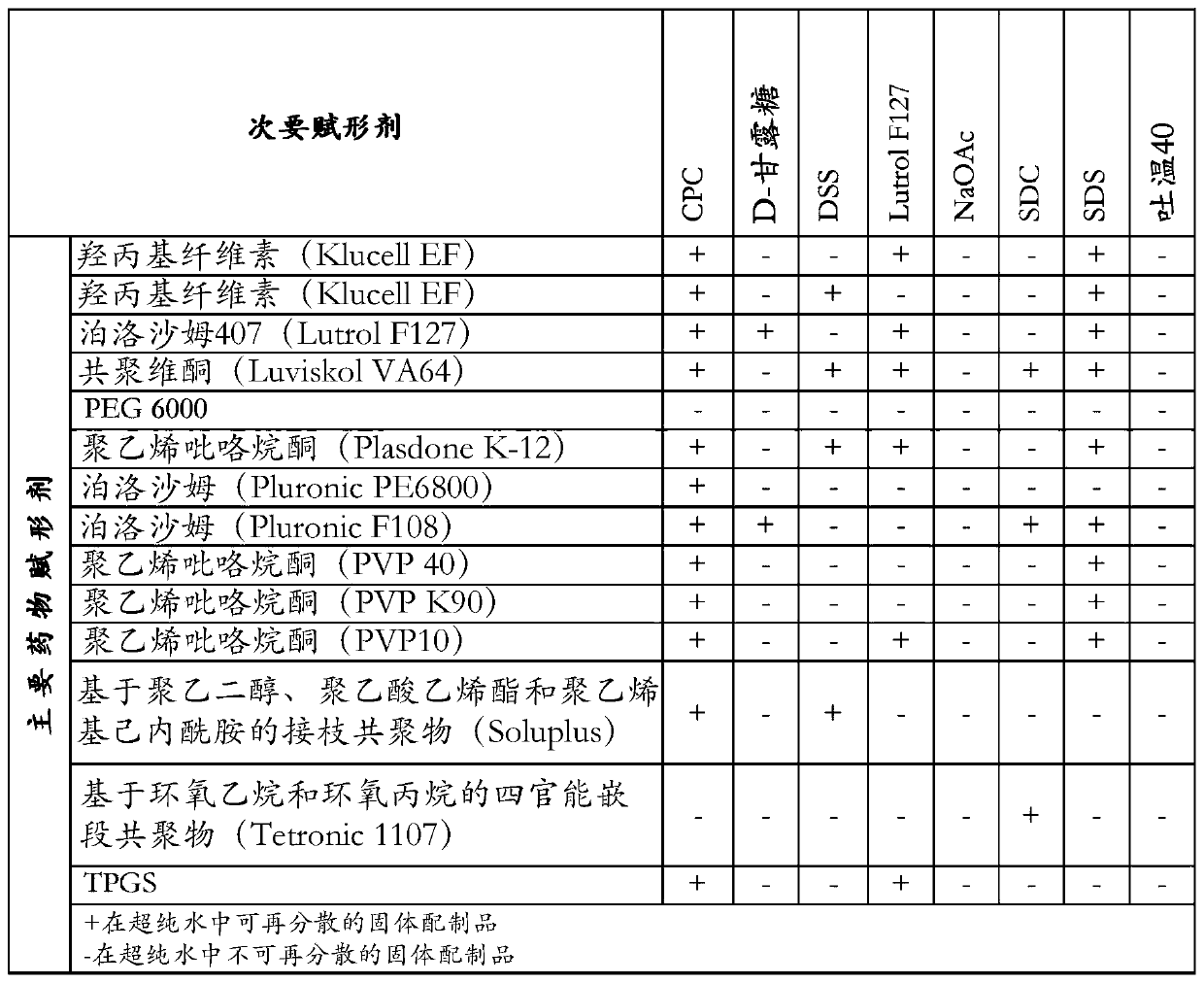
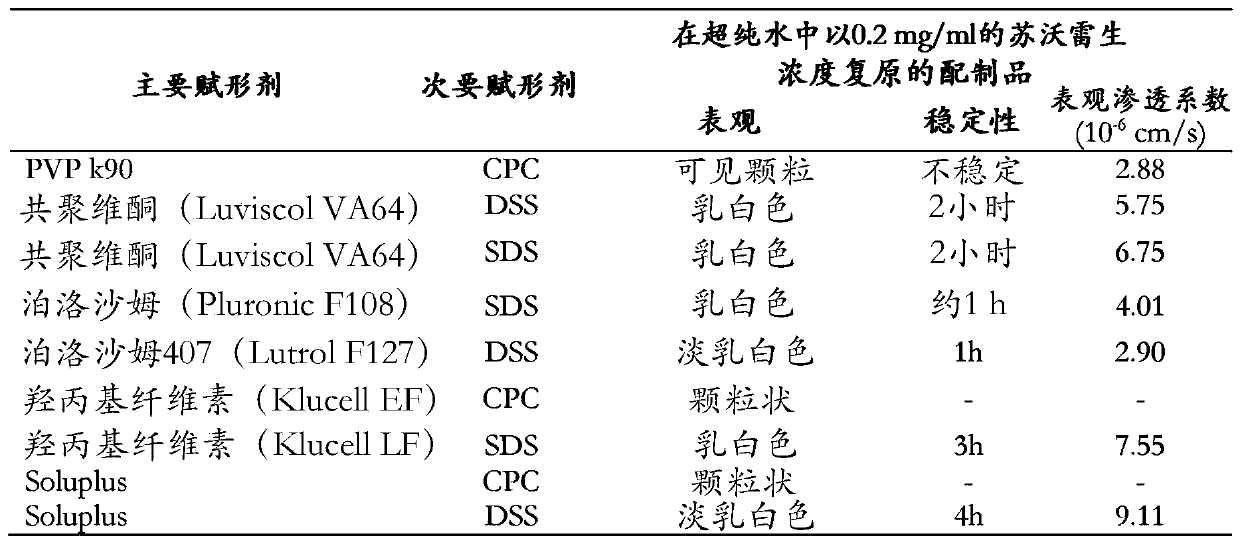
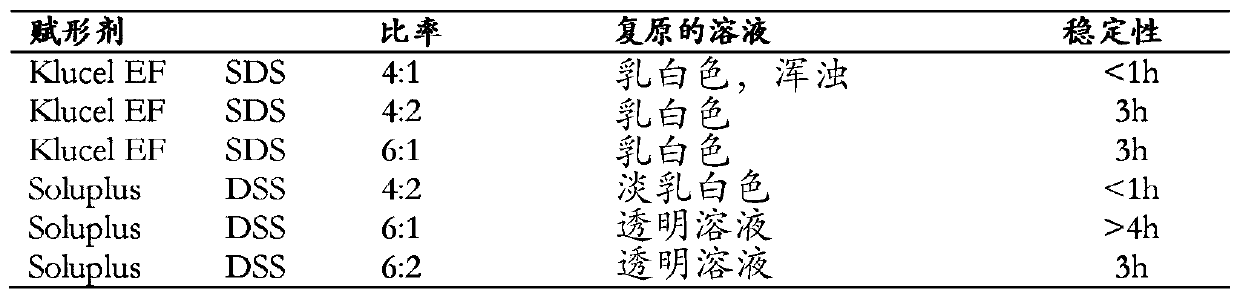
[0072]Disclosed herein are stable pharmaceutical formulations comprising suvorexan as an active compound, or a salt thereof, or a metabolite or derivative thereof, and at least one primary pharmaceutical excipient.
[0073] In one embodiment, said pharmaceutical formulation further comprises at least one secondary pharmaceutical excipient.
[0074] We have found that only selected combinations of primary and secondary pharmaceutical excipients disclosed in the present invention lead to stable pharmaceutical formulations with improved physicochemical characteristics and enhanced biological properties.
[0075] The primary pharmaceutical excipient by itself or together with the secondary pharmaceutical excipient has the function of forming a complex structure with suvorexan, or a salt thereof, or a metabolite or derivative thereof, through non-covalent secondary interactions. Secondary interactions can be formed through electrostatic interactions such as ionic interactions, hydr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com