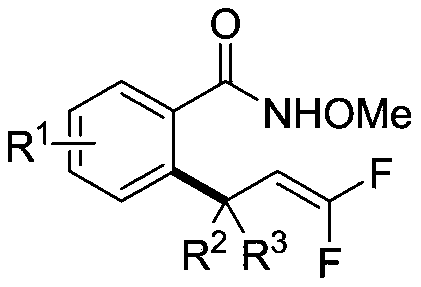
Alpha,alpha-difluoroallyl aromatic hydrocarbon compound and preparation method thereof
A technology for difluoroallyl aromatic hydrocarbons and compounds, which is applied in the field of organic chemical synthesis, can solve the problem of less organic fluorides, achieve mild reaction conditions, eliminate pre-functionalization steps, and have broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032](1) Under atmospheric atmosphere, mix 0.1mmol N-methoxy-4-methylbenzamide, 0.02mmol sodium acetate, 0.002mmol pentamethylcyclopentadienyl rhodium dichloride dimer and 0.12mmol 1-(5,5-difluoro-3-methyl-3,4-pentadienyl)benzene was sequentially added to a reaction flask filled with 0.5mL 1,2-dichloroethane to obtain a mixture;
[0033] (2) React the above mixture at room temperature (25-30° C.) for 12 h under stirring, and separate by silica gel column chromatography to obtain α,α-difluoroallyl aromatic hydrocarbon compound 1 containing all-carbon quaternary carbon centers.
Embodiment 2~46
[0035] Embodiments 2 to 46 are the same as Embodiment 1, except that:
[0036] Table 1 Examples 2-46
[0037]
[0038]
[0039]
Embodiment 47
[0041] This example is basically the same as Example 1, the difference is that in step (1), 0.1mmol N-methoxy-4-methylbenzamide, 0.2mmol sodium acetate, 0.02mmol pentamethylcyclopentadiene Alkenyl rhodium dichloride dimer and 1.2mmol 1-(5,5-difluoro-3-methyl-3,4-pentadienyl)benzene were sequentially added to 2mL 1,2-dichloroethyl The mixture was obtained in a reaction flask of alkane.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap