Atorvastatin calcium and phospholipid compound and preparation method thereof
A technology of atorvastatin calcium phospholipid and atorvastatin calcium, which is applied in the field of pharmacy and can solve complex problems (including the process of preparing tablet core, isolation coat coating process, adhesive coating process, laser drilling process and intestinal Dissolving and coating process, high energy consumption, reducing drug degradation and other problems, to achieve excellent transmembrane transport properties, good reproducibility, and promote the effect of in vivo absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
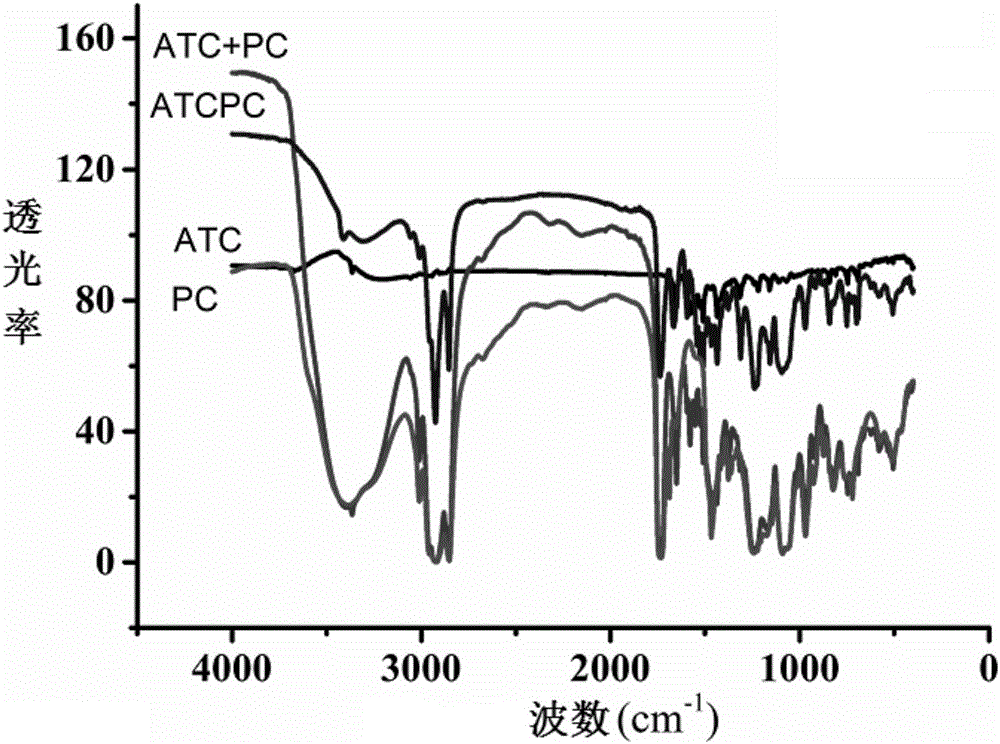
[0042] The preparation method and characterization of the atorvastatin calcium phospholipid complex of the present invention comprise the following steps:
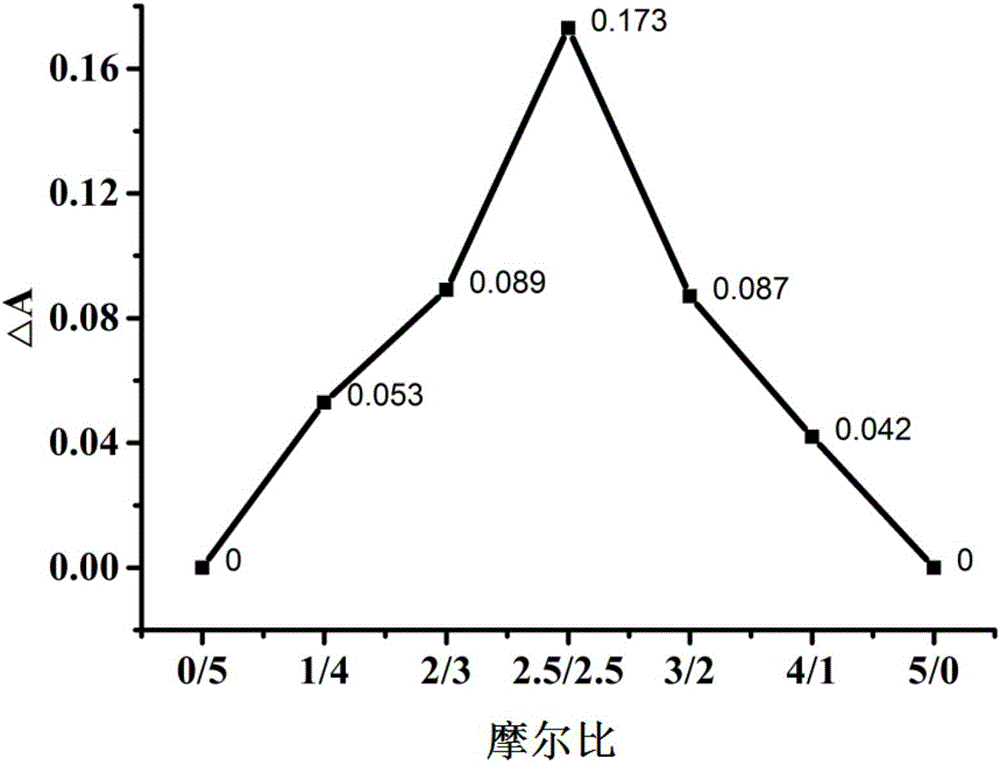
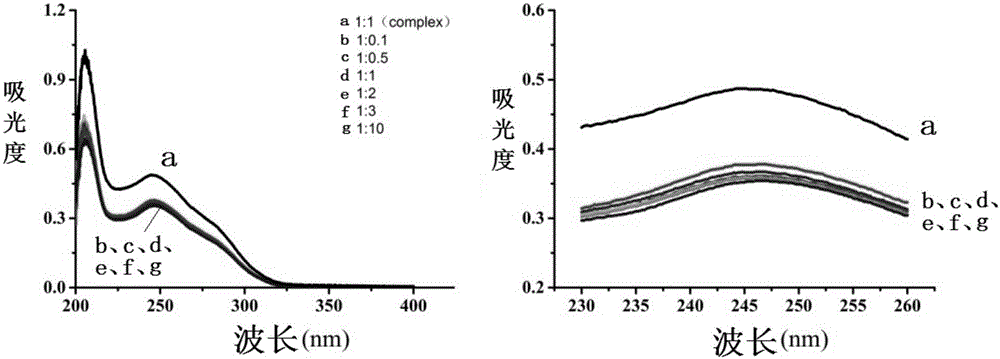
[0043](1) Prepare atorvastatin calcium with the molar ratios of atorvastatin calcium and phospholipids being 0 / 5, 1 / 4, 2 / 3, 2.5 / 2.5, 3 / 2, 4 / 1, 5 / 0 respectively Phospholipid complexes and physical mixtures and keep the drug concentration constant at 0.005mol / L. Measure the absorbance value A of atorvastatin calcium phospholipid complex and atorvastatin calcium at a wavelength of 244nm, and calculate the difference △A between the two, and plot △A against the molar ratio, and the corresponding one with the largest change in △A The mol ratio is the drug-lipid complex ratio of atorvastatin calcium-phospholipid complex.
[0044] (2) Weigh an appropriate amount of atorvastatin calcium and phospholipids in corresponding molar ratios, stir in a series of aprotic solvents at 20°C to 90°C for 0.1 to 8 hours, then carry out rotary ev...
Embodiment 1
[0051] This embodiment provides a kind of atorvastatin calcium phospholipid complex, the molar ratio that comprises atorvastatin calcium and natural soybean lecithin is 1: 1, and the total molar number of atorvastatin calcium and natural soybean lecithin is 0.005mol / L, about 0.0025mol of atorvastatin calcium and 0.0025mol of natural soybean lecithin in 1L of acetone solution, stirred at 50°C for 3 hours, then rotary evaporated at 40°C until there was no organic solvent smell, and Vacuum drying at 40°C for 24 hours to obtain the atorvastatin calcium phospholipid complex. Store at 4°C after sealing. In this embodiment, the concentration of atorvastatin calcium is 2.89 mg / ml, the concentration of phospholipid is 1.88 mg / ml, and the concentration of reactants is 4.77 mg / ml.
Embodiment 2
[0053] This embodiment provides a kind of atorvastatin calcium phospholipid complex, the molar ratio that comprises atorvastatin calcium and phosphatidylethanolamine is 1: 1, and the total molar number of atorvastatin calcium and phosphatidylethanolamine is 0.005mol / L, about 0.0025mol of atorvastatin calcium and 0.0025mol of phosphatidylethanolamine in 1L of acetone solution, stirred at 30°C for 3 hours, then rotary evaporated at 40°C until there was no organic solvent smell, and in Vacuum drying at 40° C. for 20 hours to obtain the atorvastatin calcium phospholipid complex. Store at 4°C after sealing. In this embodiment, the concentration of atorvastatin calcium is 2.89 mg / ml, the concentration of phospholipid is 1.88 mg / ml, and the concentration of reactants is 4.77 mg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com