Galactose-artemisinin and method for preparing same
A technology of galactose and artemisinin, which is applied in pharmaceutical formulations, sugar derivatives, medical preparations containing active ingredients, etc., can solve the problems of increasing reaction steps, products or environmental pollution, reducing reaction efficiency, etc. Activity, solving poor treatment selectivity, reducing the effect of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
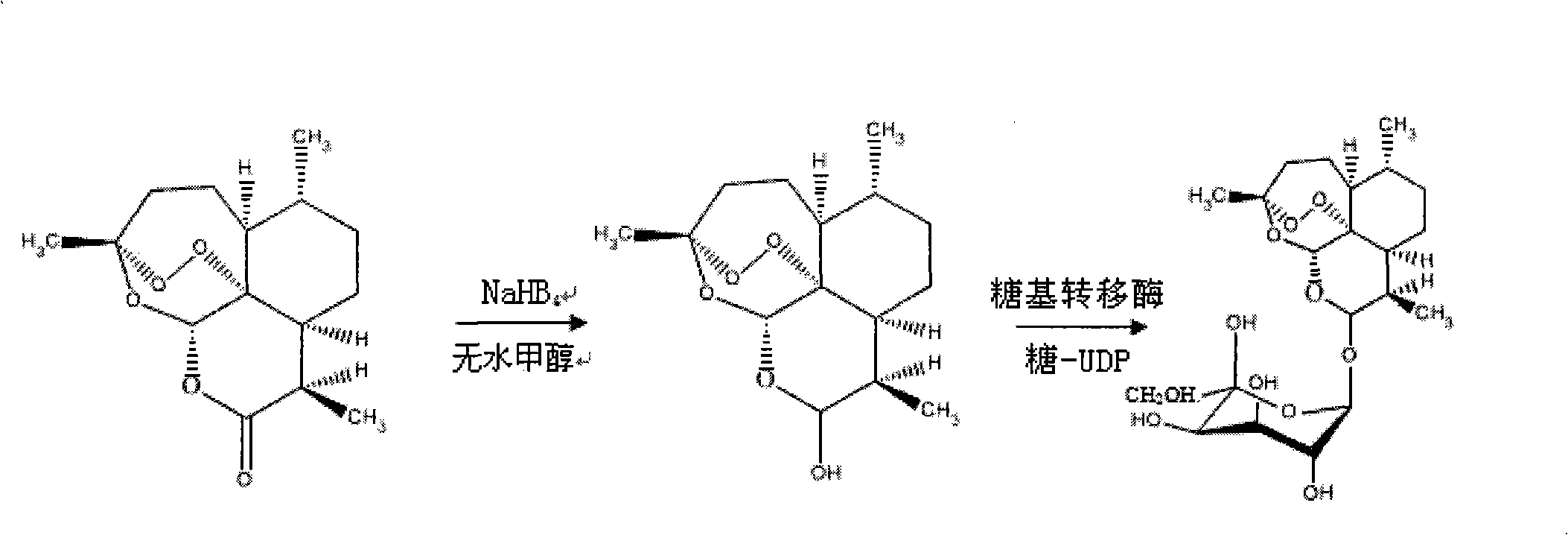
[0068] Example 1. Synthesis of receptors—reduction of artemisinin
[0069] Add 2.81g (0.01mol) artemisinin, 0.454g (0.012mol) NaHB 4 And 10mL of anhydrous methanol stirred at room temperature for 24h. The filtrate was distilled under reduced pressure to recover methanol. The residue was dissolved in 250 mL of water, then extracted three times with 50 mL of ethyl acetate, the organic layer was washed with a small amount of water, and the water washings and the water layer were combined. It was extracted with ethyl acetate, and the organic layers were combined. Evaporation of solvent, drying to obtain product - dihydroartemisinin;
Embodiment 2
[0070] Example 2. Synthesis of Donor——Preparation of UDP-Galactose
[0071] Dissolve 2.0 g of galactose in a 500 mL double-distilled water flask, and stir at a constant speed on a magnetic stirrer for 2 h. On a high-speed centrifuge at 12000rpm, centrifuge for 20min, and take the supernatant. Add 450mL of 0.4% galactose solution obtained in the previous step to 450mL of absolute ethanol, and stir gently; take out the milky white flocculent precipitate and put it in a flask, squeeze out excess water, add 450mL of deionized water, and stir until completely dissolved.
[0072] Take 20 mL of the reconstituted galactose solution, add different volumes of UDP respectively, and treat with 20 MHz, 36% ultrasonic wave for 30 min. Remove the free UDP in the solution, take 10 1.5mL Ep tubes, and add 1mL of the solution after ultrasonic treatment to each tube; turn on the vacuum centrifuge, set the temperature at 30°C, 1400rpm, and a vacuum of <20hPa, and place them in turn for 120min to...
Embodiment 3
[0073] Example 3. Enzyme-catalyzed synthesis process - preparation of galactose-artemisinin
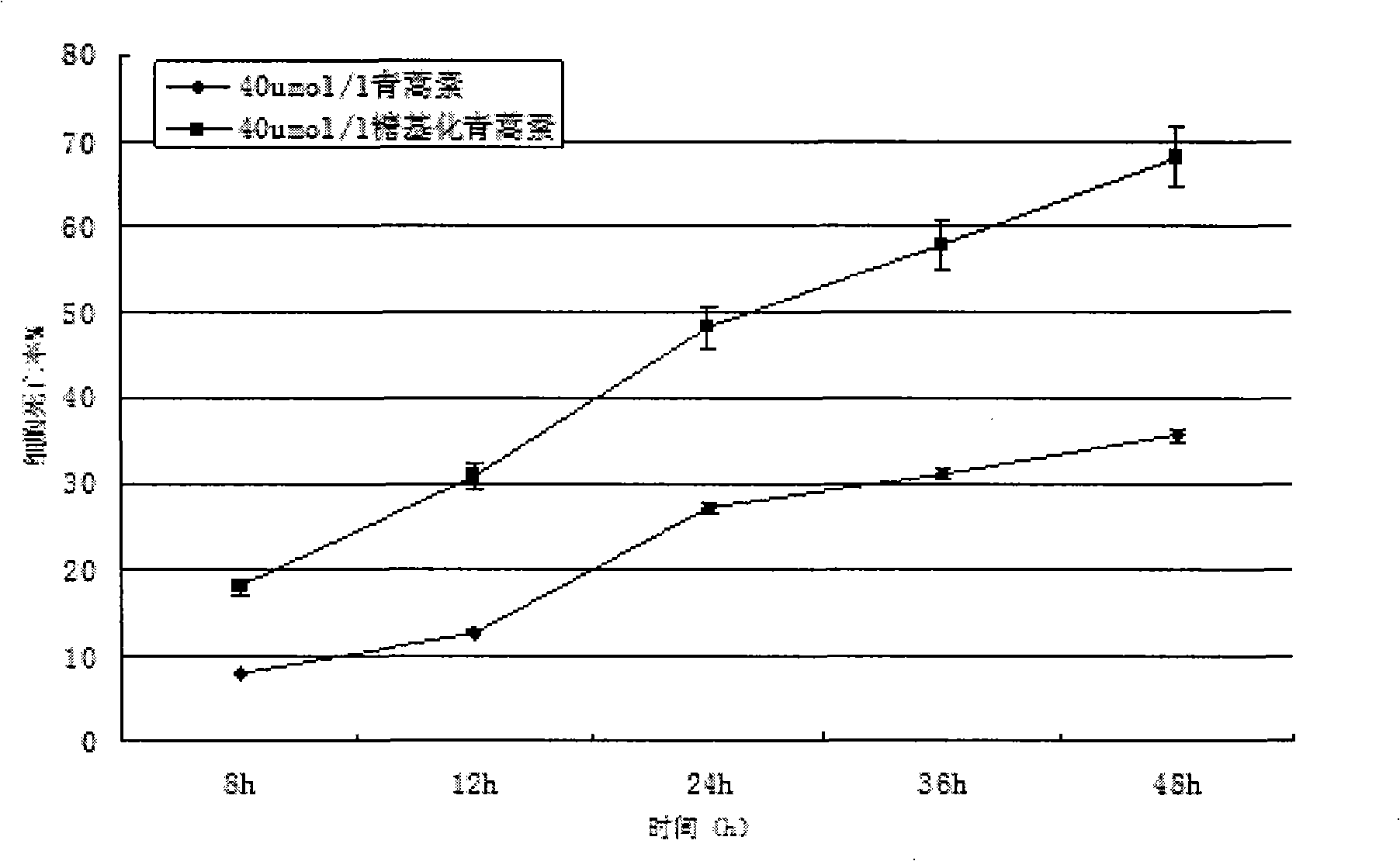
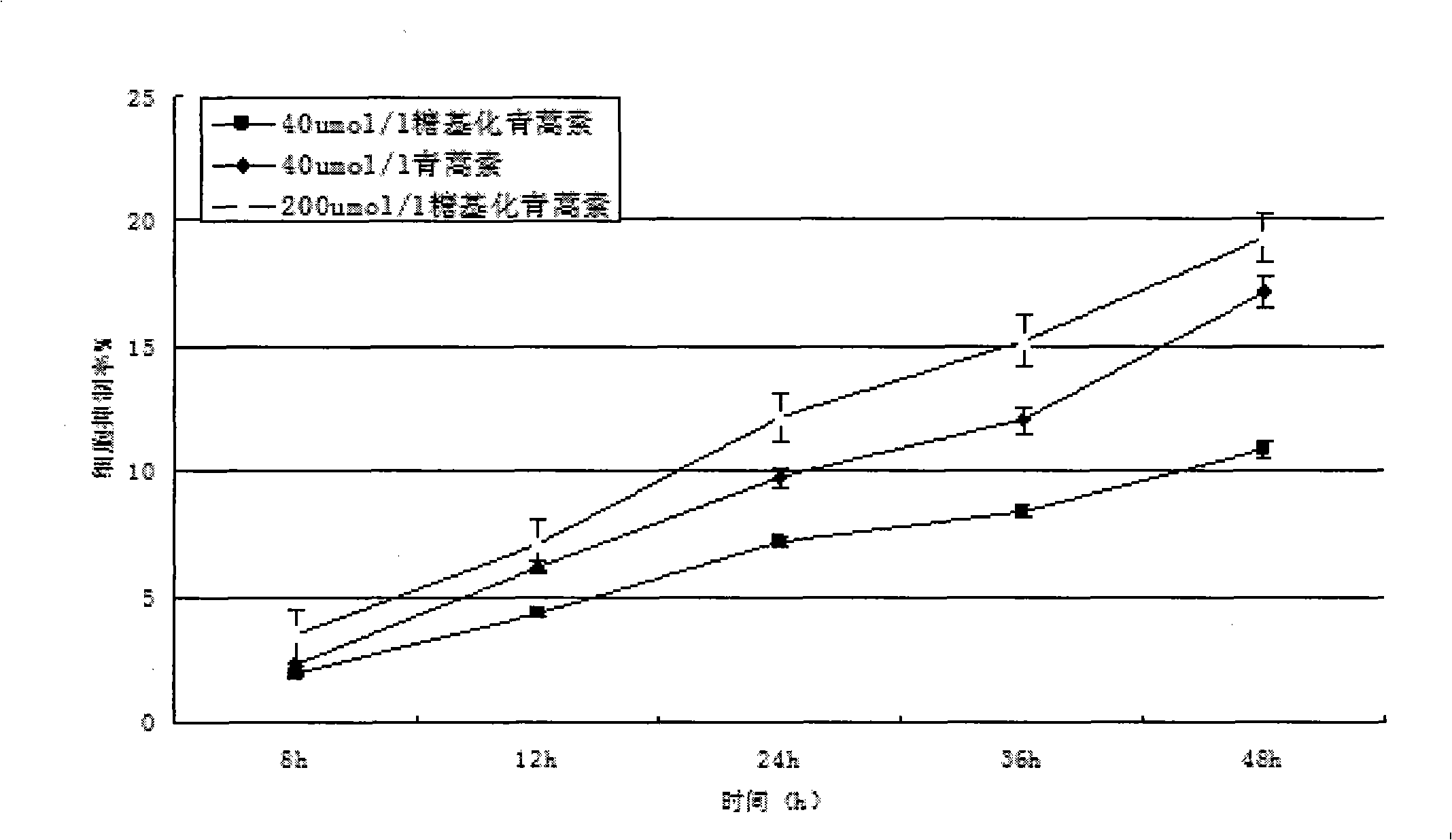
[0074] see figure 1 . Dissolve 1.4107g (equivalent to 5mmol) of dihydroartemisinin in 50mmol / L sodium azide, add 10mmol of UDP-galactose, and 5μmol of galactose glycosyltransferase, and finally add pH7.4 phosphate buffer solution diluted to 10 mL. The entire solution was incubated at a constant temperature of 37° C. for 3 days at a rotational speed of 200 r / min. Then, fully dialyze with a dialysis bag in 50 mmol / L sodium azide, pH 7.4 phosphate buffer to remove unbound galactose molecules to obtain galactose-artemisinin (or glycosylated artemisinin).
[0075] The chemical name of the galactose-artemisinin is (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3,6,9-trimethyl-3,12-oxo-12H- Pyrano[4,3-j]-1,2-benzodisepin-10-O-galactosyl, molecular formula C 21 h 34 o 10 , the molecular weight is 446.51, and the structural formula is:
[0076]
[0077] All samples were frozen in a -70...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com