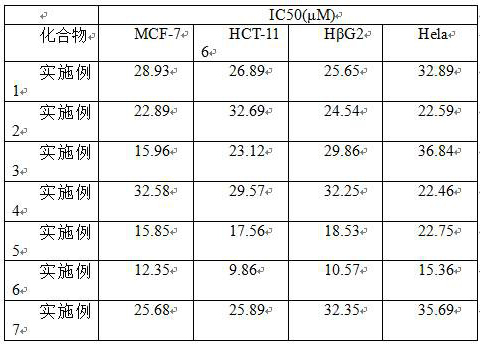
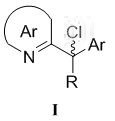
(r)-2-(α-deuterium-α-alkyl-α-aromatic) azaaryl compound and its preparation method and application
A technology of azaaryl and compound, which is applied in the field of synthesis of chiral deuterated compounds, can solve the problem of low stereoselectivity, and achieve the effects of environmental friendliness, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
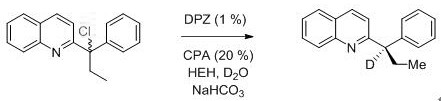
[0026] ( R )-2-(2-methyl-1-phenylpropyl-1-d) The concrete preparation steps of quinoline are as follows:
[0027]
[0028] Preparation process: Take a dry 25 mL schlenk tube, add 29.6 mg (0.1 mmol) 2-(1-chloro-2-methyl-1-phenylpropyl) quinoline, DPZ (0.35 mg, 0.001 mmol), CPA ( 1.16 mg, 0.02 mmol), HEH (38 mg, 0.15 mmol), D 2 O (200 mg, 10 mmol), sodium bicarbonate (12.6 mg, 0.15 mmol), then add 3 mL of mesitylene, cover the bottle, and degas with a vacuum pump 2-3 times at no higher than -78°C , each time for 5 to 10 min, then placed at 25°C, irradiated with a 3 W blue light, and reacted for 20 minutes. After the reaction, column chromatography separated (petroleum ether / ethyl acetate = 20~4:1, volume ratio ), concentrated by rotary evaporation, and dried in vacuo (dried at 25°C for 1 hour) to obtain 19.8 mg of white solid ( R )-2-(2-methyl-1-phenylpropyl-1-d)quinoline with a yield of 75%, an enantiomeric excess of 93%, and a deuterated rate of >99%. NMR and mass spect...
Embodiment 2
[0030] ( R )-2-(2-methyl-1-(4-(trifluoromethyl)phenyl) propyl-1-d) quinoline The specific preparation steps are as follows:
[0031]
[0032]In the present example, 2-(1-chloro-2-methyl-1-phenylpropyl)quinoline in Example 1 was used 2-(1-chloro-2-methyl-1-(4-( s trifluoromethyl)) propyl) quinoline replacement, other steps are identical with embodiment 1, obtain 22.4 mg colorless oil ( R )-2-(2-methyl-1-(4-(trifluoromethyl)phenyl)propyl-1-d)quinoline, yield 68%. The enantiomeric excess is 95%, and the deuteration rate is >99%. NMR and mass spectrometry data are: 1 H NMR (300 MHz, CDCl 3 ) δ 8.28 – 7.95 (m, 2H), 7.81 – 7.59 (m,4H), 7.51 – 7.45 (m, 3H), 7.34 (d, J = 8.4 Hz, 1H), 2.89 – 2.85 (m, 1H), 0.93– 0.85 (m, 6H); 13 C NMR (75 MHz, CDCl 3 ) Δ 162.8, 147.0, 136.5, 129.4, 129.2,128.8, 127.4, 126.9, 126.0, 125.3, 125.2, 125.2, 121.2,32.2, 21.6, 21.3; HRMS (ESI) M / Z 331.1519 (m +H + ), calc. for C 20 h 18 DF 3 N331.1527.
Embodiment 3
[0034] ( R )-2-(2-methyl-1-(2-naphthyl) propyl-1-d) The concrete preparation steps of quinoline are as follows:
[0035]
[0036] In this example, the 2-(1-chloro-2-methyl-1-phenylpropyl)quinoline in Example 1 was used 2-(1-chloro-2-methyl-1-(2-naphthyl) base) propyl) quinoline to give 22.5 mg colorless oil ( R )-2-(2-methyl-1-(2-naphthyl)propyl-1-d)quinoline in 72% yield, 88% enantiomeric excess, and >99% deuterated rate. NMR and mass spectrometry data are: 1 H NMR (300 MHz, CDCl 3 ) δ 8.13 (d, J = 8.3 Hz, 1H), 8.00 (d, J = 8.6 Hz, 1H), 7.91 (s, 1H),7.84 – 7.63 (m, 6H), 7.50 –7.33 (m, 4H), 3.00 – 2.90 (m, 1H), 0.96 (d, J =6.4 Hz, 6H); 13 C NMR (75 MHz, CDCl 3 ) Δ 163.7, 147.6, 140.4, 136.3, 133.5,132.3, 129.3, 129.1, 127.7, 127.5, 127.0, 126.8, 125.8,125.4, 121.7, 21.7, 21.5; z 313.1802 (M+H + ), calc.forC 23 h 21 DN 313.1810.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com