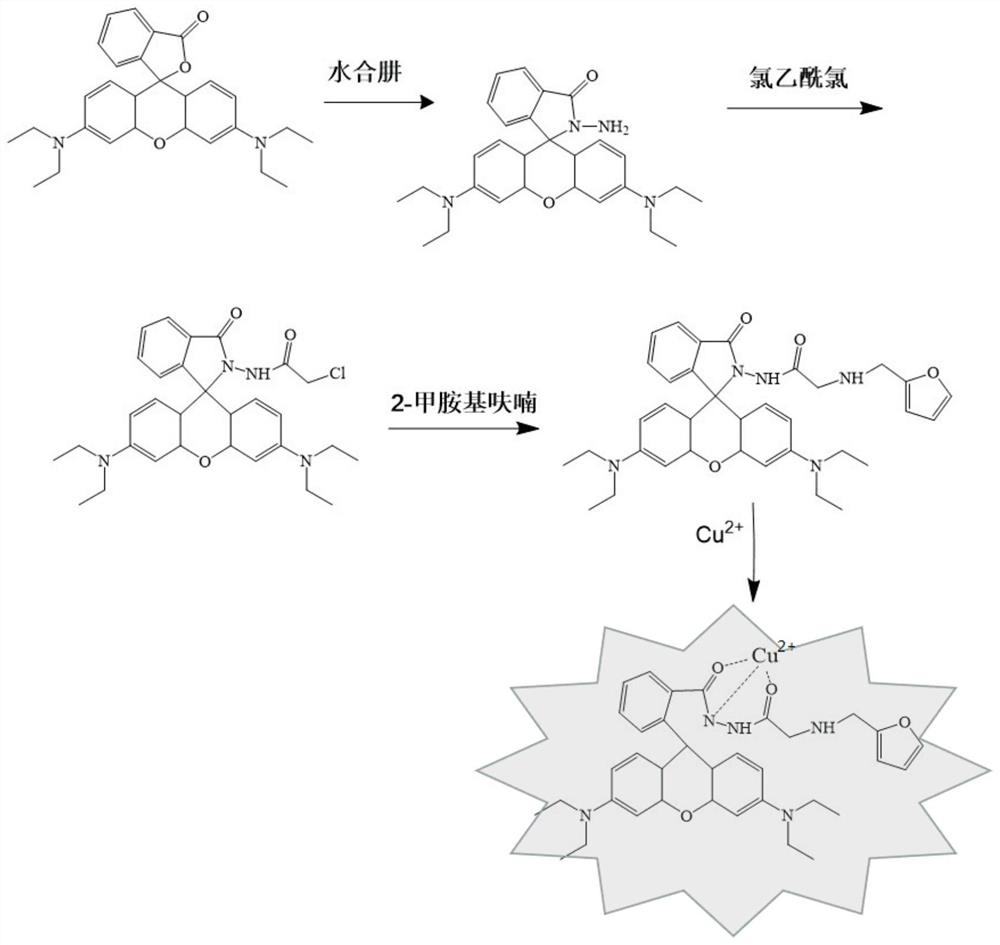
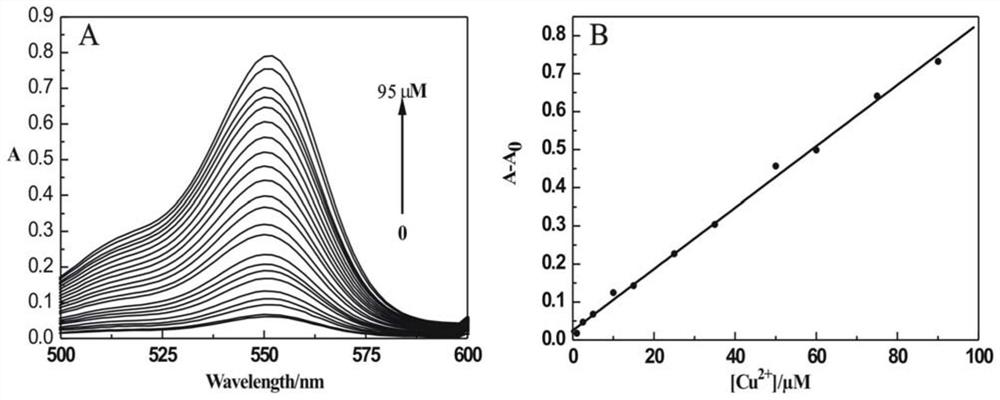
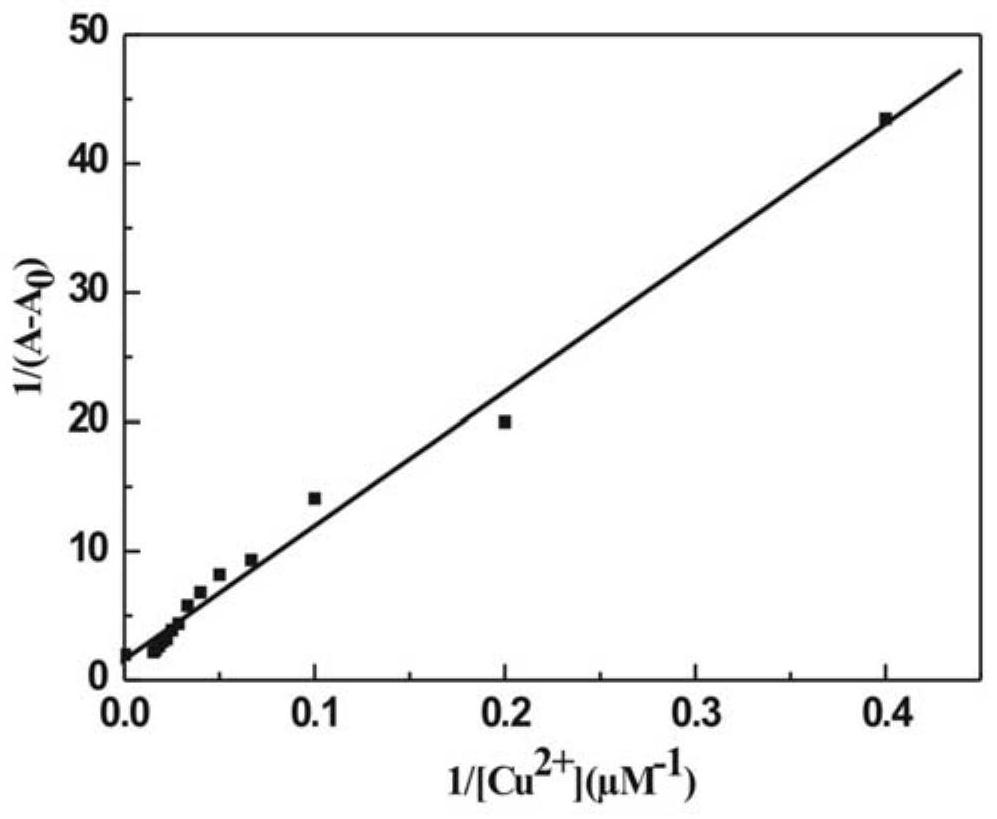
Preparation and application of aminoacylmethyl-(2-methylaminofuran)rhodamine amide derivative
A technology of methylaminofuran and aminoacylmethyl, which is applied in the field of easy preparation of rhodamine derivative optical sensing materials, and can solve the problems of low sensitivity and selectivity of optical sensing, harsh reaction conditions, and difficult purification and separation. , to achieve the effect of large molar extinction coefficient, mild reaction conditions and high specificity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Weigh 2.40g (5.00mmol) of Rhodamine B and place it in a flask, add 65mL of ethanol to dissolve it, then slowly add 5.0mL of hydrazine hydrate (98% by mass) dropwise, after reflux for 3 hours, evaporate the solvent, The residual solvent was dissolved in water, and the pH was adjusted to about 7.0. A large amount of precipitate was precipitated, and the precipitate was filtered and dried to obtain rhodamine B hydrazide.
[0029] (2) Weigh 1.438g (3.12mmol) of rhodamine B hydrazide and dissolve it in 60mL of dichloromethane, add 2.0mL (12.50mmol) of chloroacetyl chloride, and add 3.0mL of triethylamine as an acid-binding agent , reacted rapidly under ice-salt bath for 10 h, and evaporated the solvent to obtain a lavender solid crude product, and then passed through a neutral alumina column to obtain a yellow-white solid.
[0030] The molar ratio of rhodamine B hydrazide to chloroacetyl chloride is 1:4.
[0031] When neutral alumina passes through the column, the volum...
Embodiment 2
[0035] (1) Weigh 2.40g (5.00mmol) Rhodamine B and place it in a flask and add 70mL of ethanol to dissolve it, then slowly add 6.0mL of hydrazine hydrate (98% by mass) dropwise, after the reaction is refluxed for 3h, after evaporating the solvent, The residual solvent was dissolved in water, and the pH was adjusted to about 7.0. A large amount of precipitate was precipitated, and the precipitate was filtered and dried to obtain rhodamine B hydrazide.
[0036] (2) Weigh 1.438g (3.12mmol) of rhodamine B hydrazide and dissolve it in 65mL of dichloromethane, add 2.25mL (14.06mmol) of chloroacetyl chloride, and add 3.5mL of triethylamine as the acid-binding reagent, reacted rapidly under ice-salt bath for 11 h, and evaporated the solvent to obtain a lavender solid crude product, and then passed through a column of neutral alumina to obtain a yellow-white solid.
[0037] The molar ratio of rhodamine B hydrazide to chloroacetyl chloride is 1:4.5.
[0038] When neutral alumina passes th...
Embodiment 3
[0042] (1) Weigh 2.40g (5.00mmol) Rhodamine B and place it in a flask and add 60mL of ethanol to dissolve it, then slowly add 6.0mL of hydrazine hydrate (98% by mass) dropwise, after the reaction is refluxed for 4h, after evaporating the solvent, The residual solvent was dissolved in water, and the pH was adjusted to about 7.0. A large amount of precipitate was precipitated, and the precipitate was filtered and dried to obtain rhodamine B hydrazide.
[0043] (2) Weigh 1.438g (3.12mmol) of rhodamine B hydrazide and dissolve it in 60mL of dichloromethane, add 2.0mL (12.50mmol) of chloroacetyl chloride, and add 4.0mL of triethylamine as an acid-binding agent , reacted rapidly under ice-salt bath for 12h, and evaporated the solvent to obtain a lavender solid crude product, and then passed through a neutral alumina column to obtain a yellow-white solid.
[0044] The molar ratio of rhodamine B hydrazide to chloroacetyl chloride is 1:4.
[0045] When neutral alumina passes through t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com