Method for large-scale preparation of recombinant human acidic fibroblast growth factor (hFGF-1)
A technology for fibroblasts and growth factors, which can be applied in the fields of fibroblast growth factors, preparation methods of peptides, growth factors/inducing factors, etc. 1. Influence and other issues, to achieve the effect of production stability and applicability, good genetic stability, and stable expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
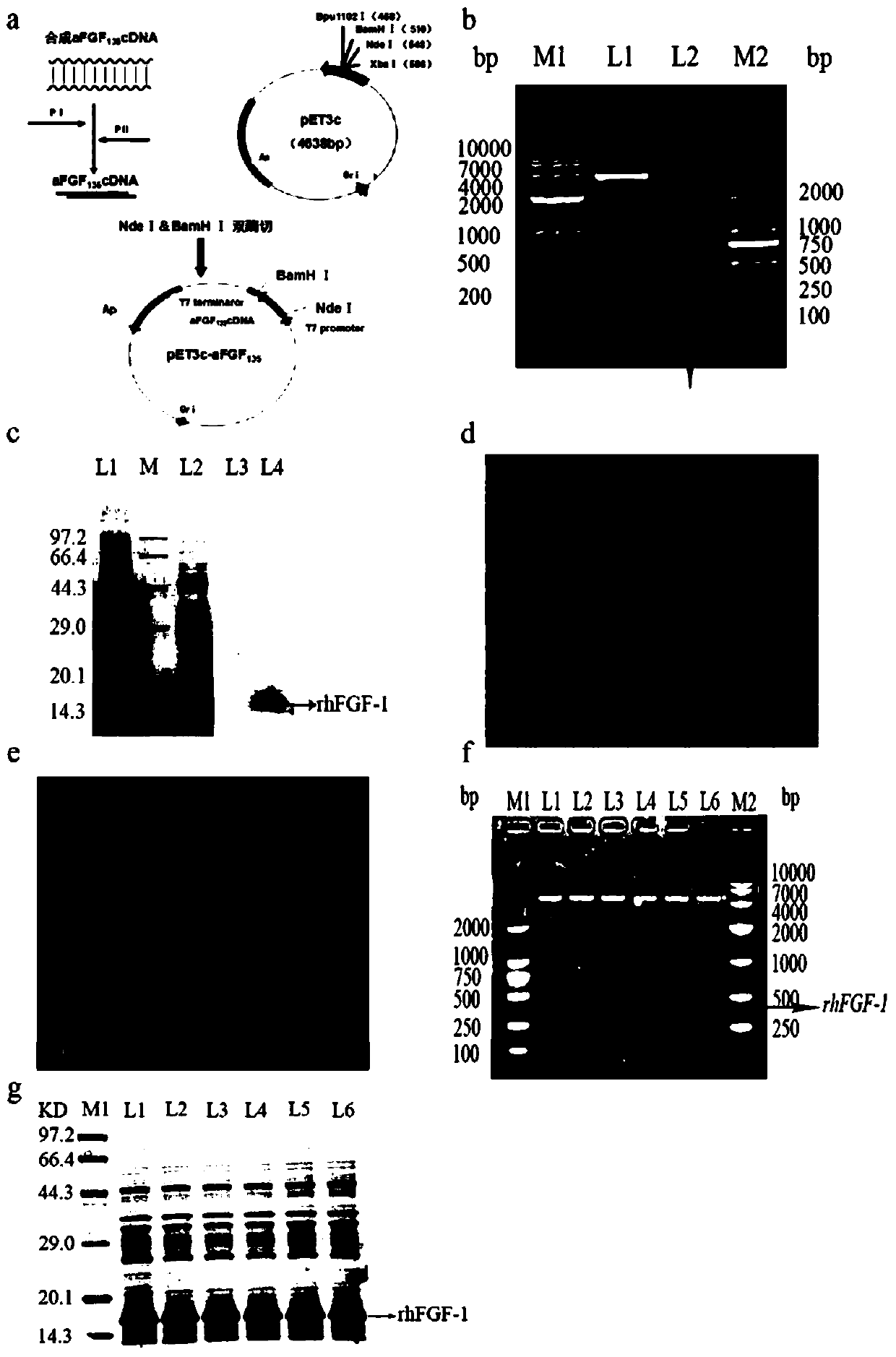
[0048] Example 1: Establishment and Identification of Engineering Bacteria Containing pET3c / rhFGF-1
[0049] 1.1 Construction and transformation of recombinant plasmid pET3c / rhFGF-1
[0050] In this example, codon optimization is performed on the gene sequence of hFGF-1 (GenBank, NM_001144892.2), which mainly includes excising 1-13 signal peptide amino acids at the N-terminus and 6 amino acids at the N-terminus of the mature protein (excluding the N-terminus). Initial amino acid methionine (M) total 19 amino acids to obtain hFGF-1 135 , the amino acid sequence of which is shown in SEQ ID NO:1. hFGF-1 135 Introduce NdeI and BamHI restriction enzyme sites (indicated in italics and underline) at both ends of the template, and design upstream and downstream primers: upstream primer PI: 3'; downstream primer PII: 3', for PCR amplification. The hFGF-1 gene and pET3c empty vector were treated with NdeI and BamHI enzymes respectively, digested at 37°C for 3 hours, and the corre...
Embodiment 2
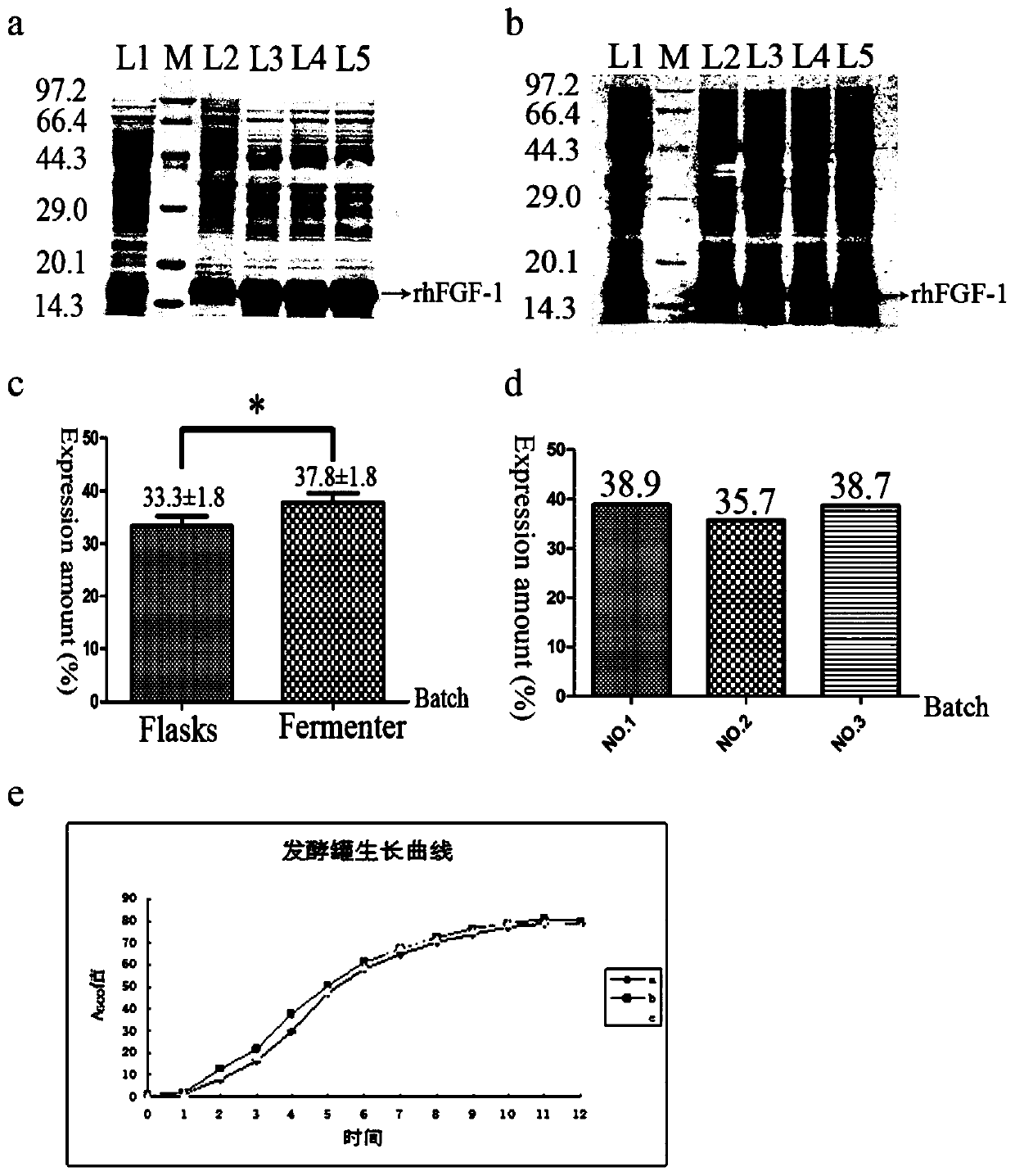
[0057] Embodiment 2: engineering bacterium stability test
[0058] In order to study the stability of the engineered bacteria, this embodiment identified the stability of the plasmid and the expression stability of rhFGF-1 of the 10th, 20th, 30th, 40th, and 50th generation engineered bacteria during the propagation process.
[0059] Pick a single colony from the solid LB plate containing ampicillin sodium and inoculate it on the slant of the LB test tube containing ampicillin sodium, culture at 37°C for 12 hours as one generation, pick a little bacterial lawn and continue to streak and subculture until the 50th generation. During this process, appropriate dilution of the bacterial solution was taken every 10 generations and spread on the LB plate without ampicillin sodium. Incubate overnight at 37°C on solid plates and count.
[0060] The primary and 10th, 20th, 30th, 40th, and 50th generation engineering bacteria were inoculated into LB liquid medium containing 100 μg / ml amp...
Embodiment 3
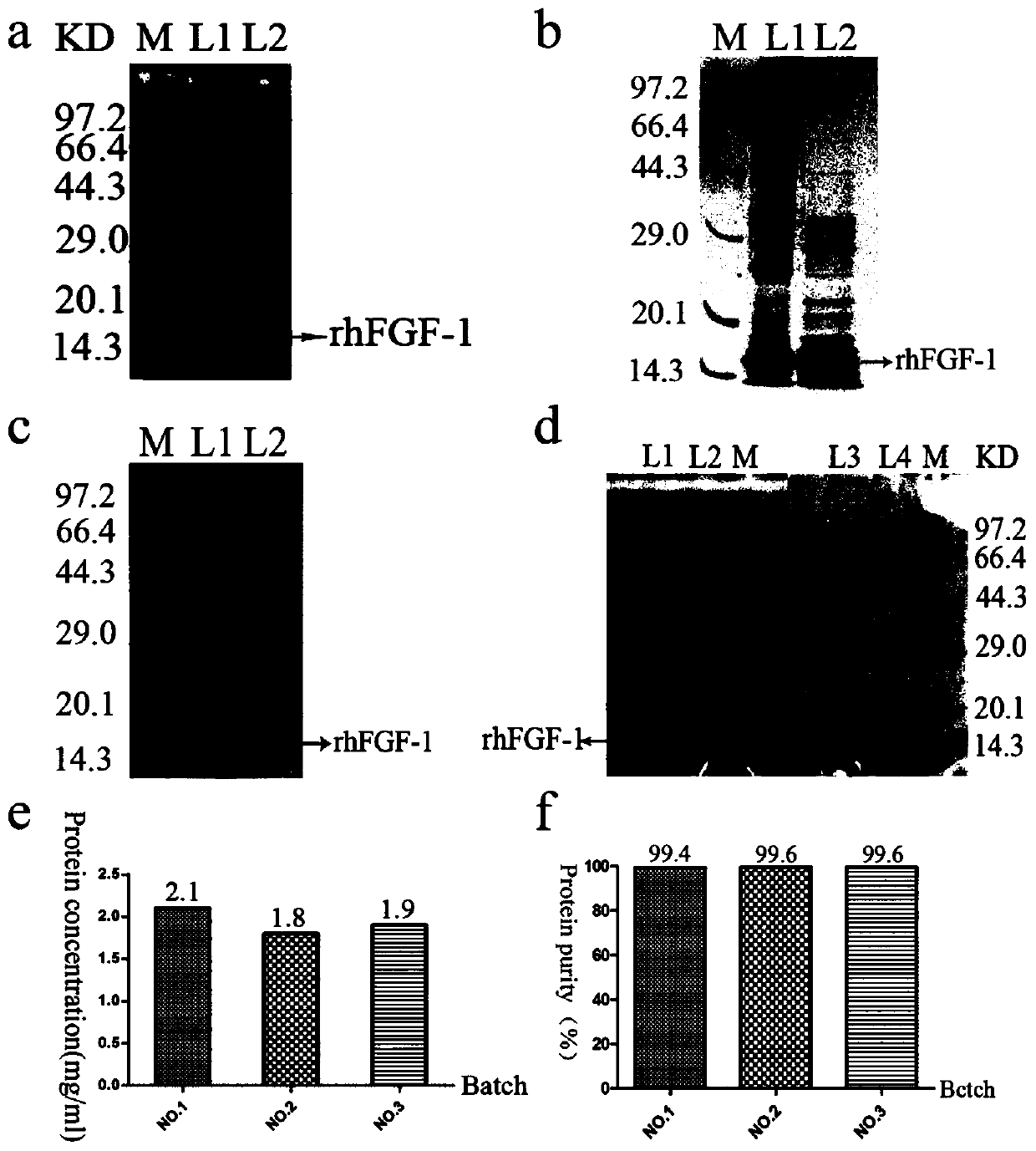
[0065] Embodiment 3: Optimization of rhFGF-1 fermentation process
[0066] 3.1 Research and verification of rhFGF-1 small-scale fermentation factors
[0067] In this example, the factors and parameters in the rhFGF-1 shake flask fermentation process were first explored, including induction time, inducer concentration, induction timing, cultivation and induction temperature, pH value, dissolved oxygen, glucose concentration, chlorination The ammonium concentration was repeated three times to determine the optimal fermentation conditions.
[0068] Specifically: the engineering bacteria BL21 (DE3) plysS-pET3C / rhFGF-1, with 1:100 (V / V), inoculated in the 30ml liquid LB culture medium that contains ampicillin sodium (final concentration 100 μ g / ml), 37 Cultivate with shaking at 150rpm overnight; replant in a 250ml Erlenmeyer flask with 30ml of liquid LB medium at a ratio of 1:100, and culture with shaking at 200rpm for 12 hours at 37°C; put the bacterial solution in the logarithmi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap