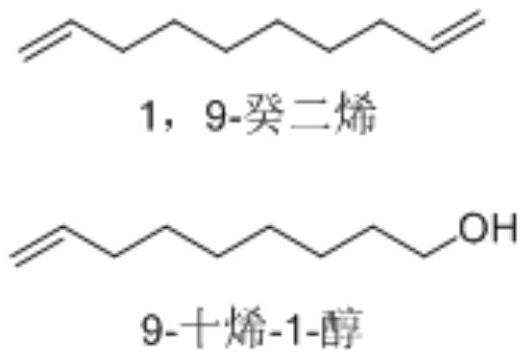
Method for co-producing 1,9-decadiene and 9-decen-1-alcohol in fixed bed reactor
A fixed-bed reactor, decadiene technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of difficult industrial production, expensive catalysts, complex reaction conditions, etc., and achieve convenient post-processing. , Avoid the use of expensive catalysts, the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
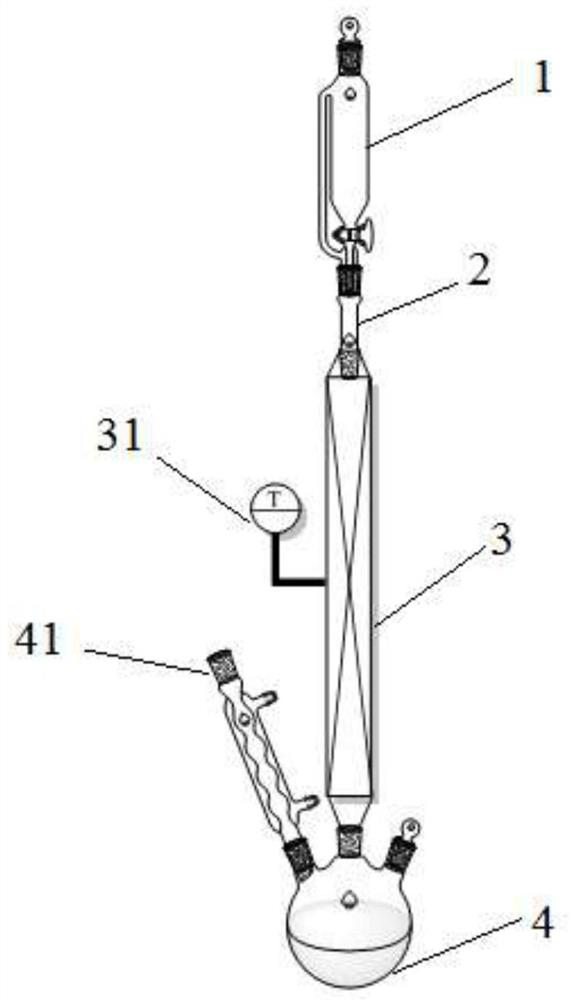
[0047] Example 1, a reaction device, such as figure 1 As shown, it includes a dripping device 1, a connecting pipe 2, a fixed bed reactor 3 and a collection container 4 connected in sequence from top to bottom;
[0048] The collection container 4 is provided with a spherical condensation pipe 41, and the effect of the spherical condensation pipe 41 is to condense the discharge of gas discharged from the bottom of the fixed bed reactor 3; the fixed bed reactor 3 is heated in parallel with electric coils, and the fixed bed reactor 3 is provided There is a thermocouple 31, and this thermocouple 31 is used to display the temperature in the fixed-bed reactor 3 in real time. The purpose of controlling the temperature in the fixed-bed reactor 3 is to realize the pyrolysis reaction of the esterification intermediate product.
[0049] A filler is arranged in the fixed bed reactor 3, and the filler is a glass Raschig ring filler with a particle size of 3±0.5mm.
[0050] The esterified...
Embodiment 1-1
[0061] Example 1-1, a fixed bed reactor mainly produces 1,9-decadiene and by-products 9-decen-1-ol, the following steps are carried out in sequence:
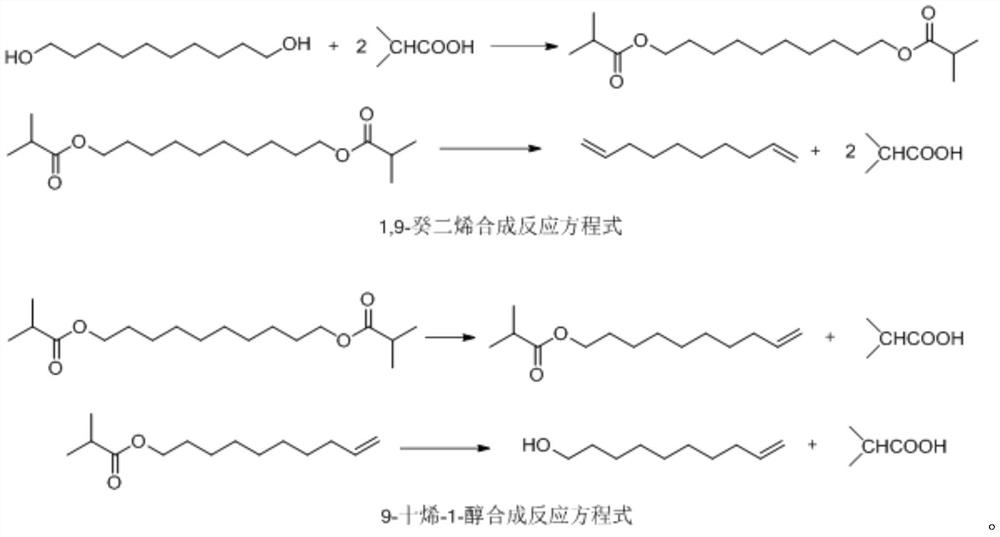
[0062] 1) Mix 34.8g (0.2mol) of 1,10-decanediol and 70.4g (0.8mol, 4eq) of isobutyric acid to form a reaction solution, put it in a 250mL reaction bottle, and raise the temperature to 150°C for esterification. The water evaporated from the esterification reaction is discharged in real time; after 4 hours of the esterification reaction, no more water is produced, and the temperature is raised to 200°C to evaporate the isobutyric acid, and the evaporated isobutyric acid is recovered; when the isobutyric acid is no longer evaporated , the temperature was lowered to room temperature to obtain an esterification intermediate;
[0063] 2), put the esterified intermediate product into the dripping device 1, and the esterified intermediate product is added dropwise (about 15g / h dropping speed) through the connecting pipe 2 and then added...
Embodiment 1-2~ Embodiment 1-6
[0068] Example 1-2~Example 1-6, a fixed bed reactor mainly produces 1,9-decadiene and a by-product 9-decen-1-ol method, changing the reaction of the esterification intermediate product in the fixed bed The reaction space velocity in device 3, the reaction temperature in fixed-bed reactor 3, all the other are equal to embodiment 1-1; Reaction parameter and obtained result are described in following table 1.
[0069] Table 1. Main production of 1,9-decadiene and by-product of 9-decen-1-ol
[0070]
[0071]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| voidage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com