Method for synthesizing 1,9-decadiene in fixed bed reactor
A fixed-bed reactor and decadiene technology, applied in chemical instruments and methods, preparation of organic compounds, hydrocarbons, etc., can solve problems such as expensive catalysts, complex reaction conditions, and complex production conditions, so as to avoid expensive Catalyst, high reaction selectivity, and high raw material utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
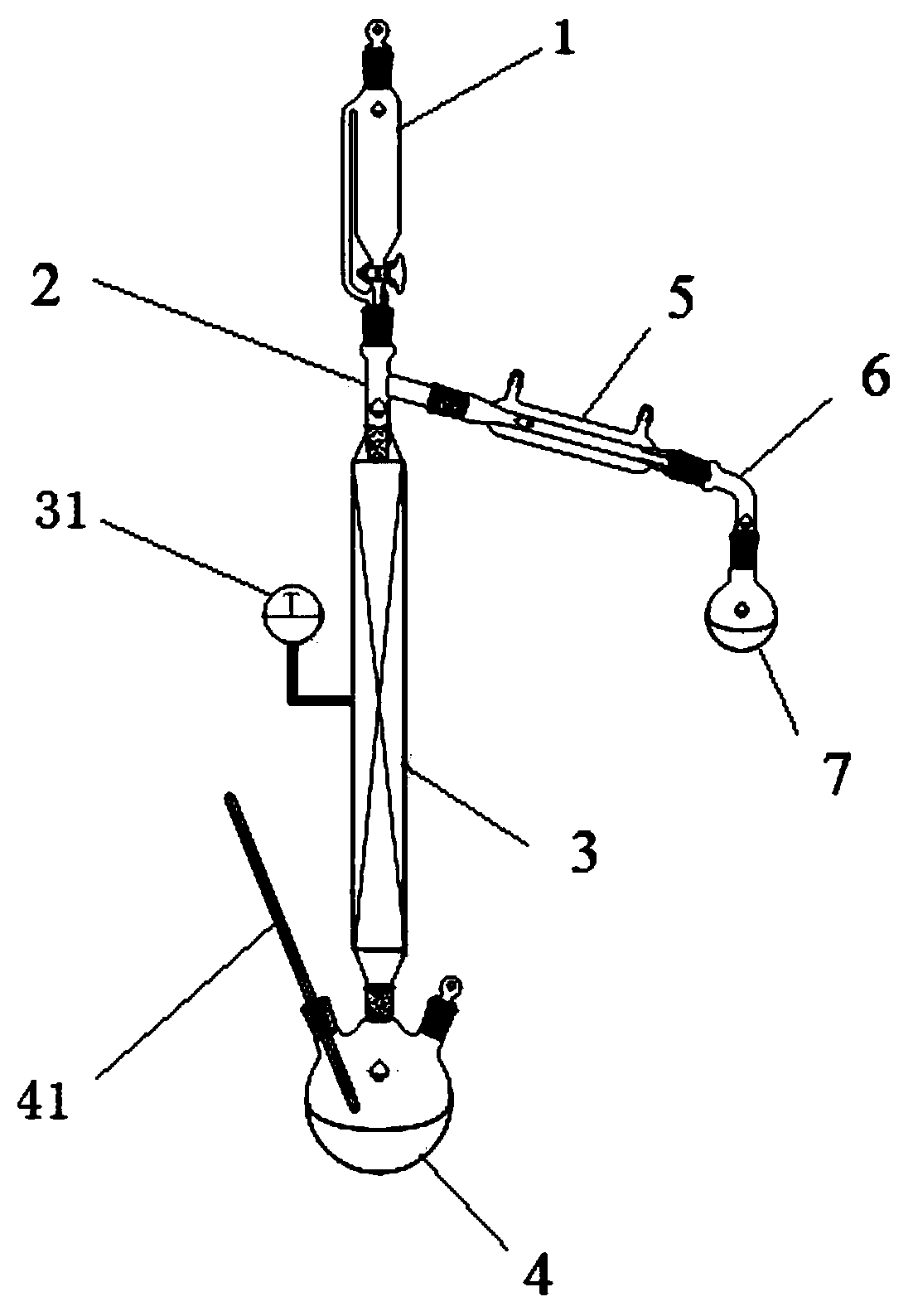
[0046] Example 1, a reaction device, such as figure 1 As shown, it consists of a dripping device 1, a tee pipe 2, a fixed bed reactor 3, a reaction vessel 4, a condenser 5, a horn tube 6, and a collection vessel 7;
[0047] The reaction vessel 4 is heated with an oil bath, and the reaction vessel 4 is provided with a thermometer 41, which is used to display the temperature in the reaction vessel 4 in real time; the purpose of controlling the temperature in the reaction vessel 4 is to maintain a certain temperature and prevent the product 1 from 9-decadiene is left, so that the product is collected as much as possible at the upper end of the fixed-bed reactor 3; the utilization rate of raw materials is improved.
[0048] The fixed bed reactor 3 is heated in parallel with electric coils, and the fixed bed reactor 3 is provided with a thermocouple 31, which is used to display the temperature in the fixed bed reactor 3 in real time; the purpose of controlling the temperature in th...
Embodiment 1
[0058] Embodiment 1, a method for synthesizing 1,9-decadiene in a fixed bed reactor, the following steps are carried out successively:
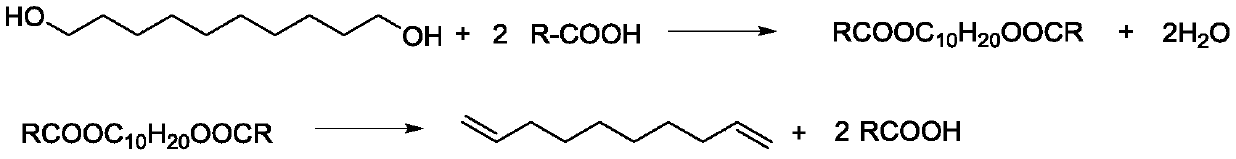
[0059] 1) Mix 34.8g (0.2mol) of 1,10-decanediol and 153.8g (0.6mol, 3eq) of palmitic acid in a 250mL reaction bottle as a reaction solution, and heat up to 150°C for esterification reaction. The water evaporated from the chemical reaction is discharged from the reaction bottle in real time;
[0060] After 3 hours of the esterification reaction, no more water was discharged; therefore, the esterification reaction was terminated to obtain an esterification intermediate product;
[0061] 2), the esterification intermediate product is put into the dripping device 1, the esterification intermediate product is kept at a dropping temperature of 70°C, and the dripping speed of 40ml / h flows through the three-way pipe 2 and then enters the fixed bed reactor 3 for esterification. Cracking reaction; fixed bed reactor 3 filler is γ-alumina (particle size...
Embodiment 2
[0071] Embodiment 2, a method for synthesizing 1,9-decadiene in a fixed-bed reactor, the following steps are carried out successively:
[0072] 1), with embodiment 1 step 1);
[0073] 2), the temperature in the fixed-bed reactor 3 is changed from 350°C to 330°C, and the temperature in the reaction vessel 4 is changed from 180°C to 200°C; the rest are equal to step 2) of Example 1;
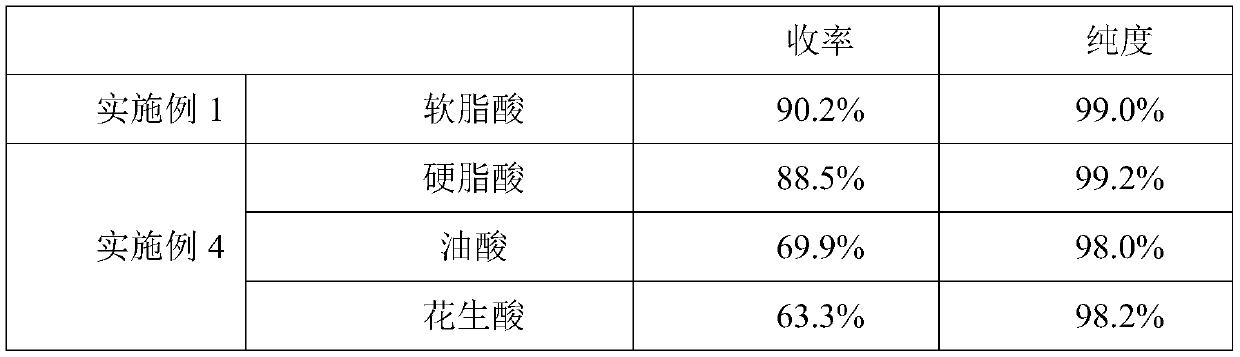
[0074] 3), the same as step 3) of Example 1; 22.6 g of 1,9-decadiene product (purity 98.9%) was obtained, and the yield was 81.8%;
[0075] 4), the same as step 4) of Example 1; 22.4 g of 1,9-decadiene product (purity: 98.8%) was obtained, and the yield was 81.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| voidage | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com