Preparation method and kit to prepare hematogenous female autologous germline stem cells and application of kit
A technology of reproductive stem cells and somatic cells, applied in the field of biomedicine, can solve the problems of time-consuming isolation and culture, and the scarcity of acquisition.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0293] The preparation of embodiment 1 kit
[0294] It is operated in a safe operation bench with a cleanliness level of 10-100, and is prepared under a low temperature of 4-10 degrees.
[0295] In 500ml of RPMI 640 culture medium, add:
[0296] 5% Serum Replacement (KSR);
[0297] EPO 10ng / ml;
[0298] M-GSF 10 ng / ml;
[0299] G-GSF 150ng / ml;
[0300] IL-3 10 ng / ml;
[0301] IL-6 10 ng / ml;
[0302] SCF 10 ng / ml;
[0303] bFGF 100 ng / ml;
[0304] Nanog 30ng / ml;
[0305] Oct4 30 ng / ml;
[0306] Sox2 20ng / ml;
[0307] c-myc 20 ng / ml.
[0308] Fully dissolve, then filter and sterilize through a filter with a pore size of 0.22 microns, and prepare cell culture solution O1.
[0309] In 500ml of M2 culture medium, add:
[0310] 5% Serum Replacement (KSR);
[0311] Nanog 30ng / ml;
[0312] Oct4 30 ng / ml;
[0313] Sox2 10 ng / ml;
[0314] c-myc 20 ng / ml;
[0315] DDX4(Mvh) 30 ng / ml;
[0316] Dazl 30ng / ml;
[0317] bFGF 100 ng / ml;
[0318] EGF 10 ng / ml;
[0319] IL-...
Embodiment 2
[0364] Example 2 Preparation of blood-derived female autologous reproductive stem cells
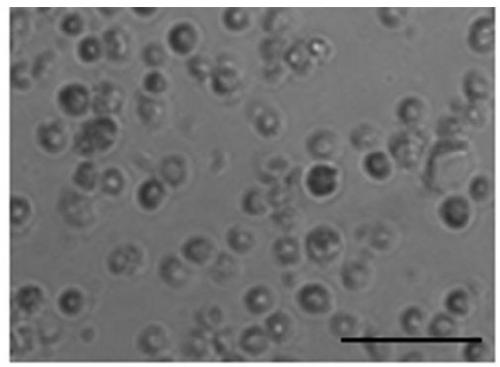
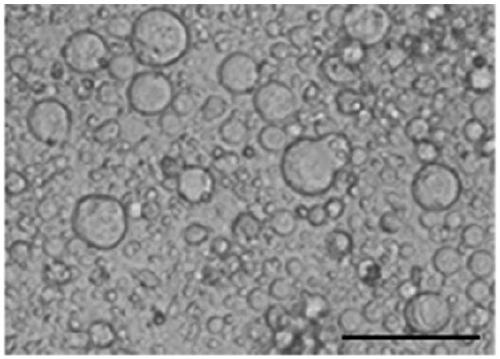
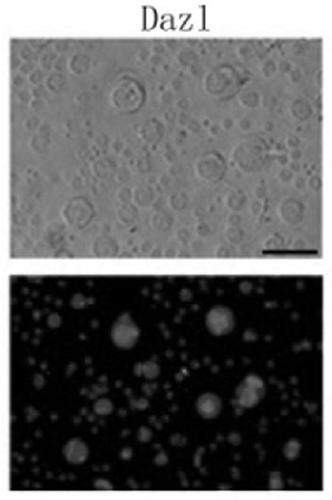
[0365] 50ml of female venous blood (from volunteers) was extracted, and the venous blood was centrifuged using conventional Ficoll centrifugal separation technology to obtain blood mononuclear cells. The obtained mononuclear cells were cultured at a density of 5x106 with the cell culture solution O1 prepared in Example 1 in an incubator at 37°C and 5% CO2 for 3 days; then replaced with the cell culture solution prepared in Example 1 O2, continue to treat mononuclear cells with 5x10 6 The density is cultivated for 6-9 days; then use the cell culture solution O3 prepared in Example 1, and continue to mononuclear cells with 5×10 6 density for 6-9 days. Gently pipette the cells cultured in cell culture medium O1, cell culture medium O2 and cell culture medium O3 to completely suspend the cells, collect the cells in a centrifuge tube, perform centrifugation, and then use normal saline to dis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com