Pharmaceutical composition for treating acute myelogenous leukemia
A composition and leukemia technology, which is applied in the field of pharmaceutical compositions for the treatment of leukemia, can solve problems such as no reports of combination therapy for leukemia, and achieve excellent results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
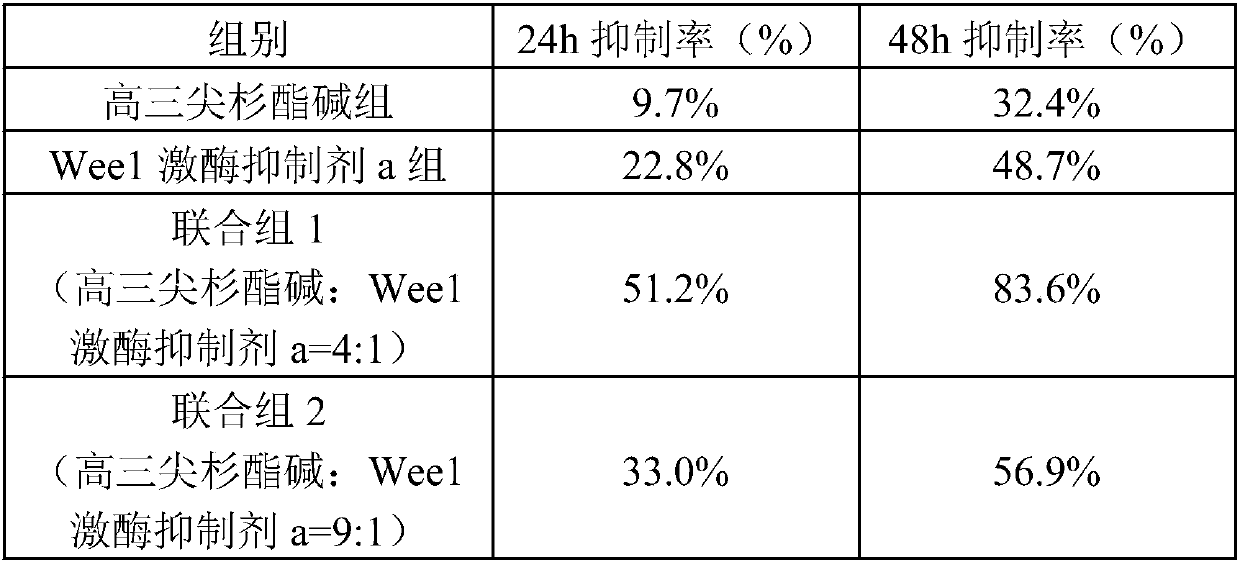
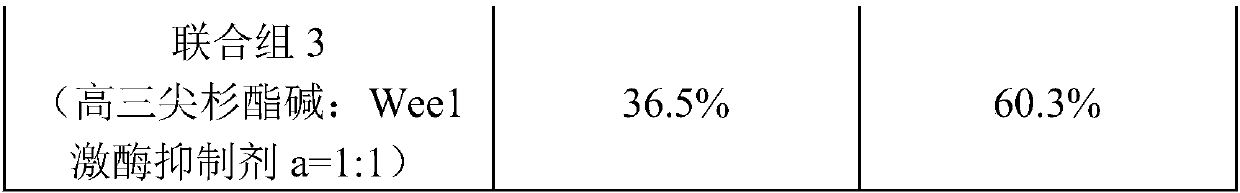
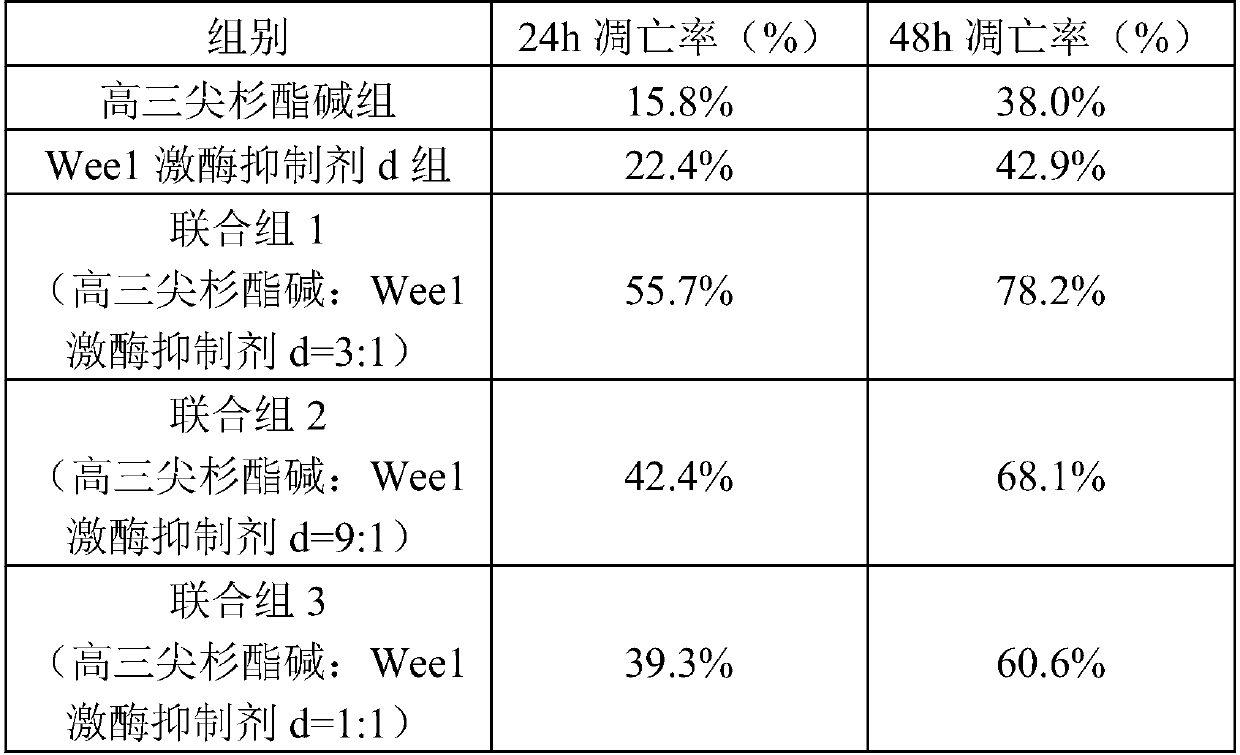
[0044] Weigh homoharringtonine 1.6g, 2-methyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(4-(4-methylpiperazine- 1-yl)phenylamino)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one 0.4g, tartaric acid 1g were dissolved in an appropriate amount of hot water for injection, and then tartaric acid solution was slowly added Homoharringtonine and 2-methyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(4-(4-methylpiperazin-1-yl) In the mixed solution of phenylamino)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one, stir constantly to dissolve all the active ingredients, then add 10g of lactose and 15g of mannitol, and stir to dissolve , adjust the pH to 4 with 4% NaOH, add water for injection to 1000ml, filter aseptically, pack the filtrate into sterilized control vials according to 1ml / branch, freeze-dry in a freeze dryer for 24h, and press the cap. Sterilized butyl rubber stoppers, rolled aluminum caps, inspected and packaged.
Embodiment 2
[0046] Weigh homoharringtonine 1g, 2-methyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(4-(4-methylpiperazine-1 -yl)phenylamino)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one 1g and tartaric acid 1g were respectively dissolved in an appropriate amount of hot water for injection, and then the tartaric acid solution was slowly added to Gao Sanjian phenetine and 2-methyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(4-(4-methylpiperazin-1-yl)phenyl In the mixed solution of amino)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one, stir continuously to dissolve all the active ingredients, then add 10g of lactose and 15g of mannitol, stir to dissolve, and use Adjust the pH to 4 with 4% NaOH, add water for injection to 1000ml, filter aseptically, pack the filtrate into sterilized controlled vials according to 1ml / vial, freeze-dry in a freeze dryer for 24 hours, and the gland has been sterilized Butyl rubber stoppers, rolled aluminum caps, inspection, and packaging.
Embodiment 3
[0048] Weigh homoharringtonine 1.8g, 2-methyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(4-(4-methylpiperazine- 1-yl)phenylamino)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one 0.2g, tartaric acid 1g were dissolved in appropriate amount of hot water for injection, and then tartaric acid solution was slowly added Homoharringtonine and 2-methyl-1-(6-(2-hydroxypropan-2-yl)pyridin-2-yl)-6-(4-(4-methylpiperazin-1-yl) In the mixed solution of phenylamino)-1H-pyrazolo[3,4-d]pyrimidin-3(2H)-one, stir constantly to dissolve all the active ingredients, then add 10g of lactose and 15g of mannitol, and stir to dissolve , adjust the pH to 4 with 4% NaOH, add water for injection to 1000ml, filter aseptically, pack the filtrate into sterilized control vials according to 1ml / branch, freeze-dry in a freeze dryer for 24h, and press the cap. Sterilized butyl rubber stoppers, rolled aluminum caps, inspected and packaged.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com