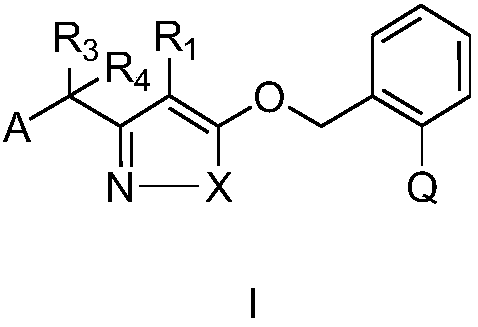
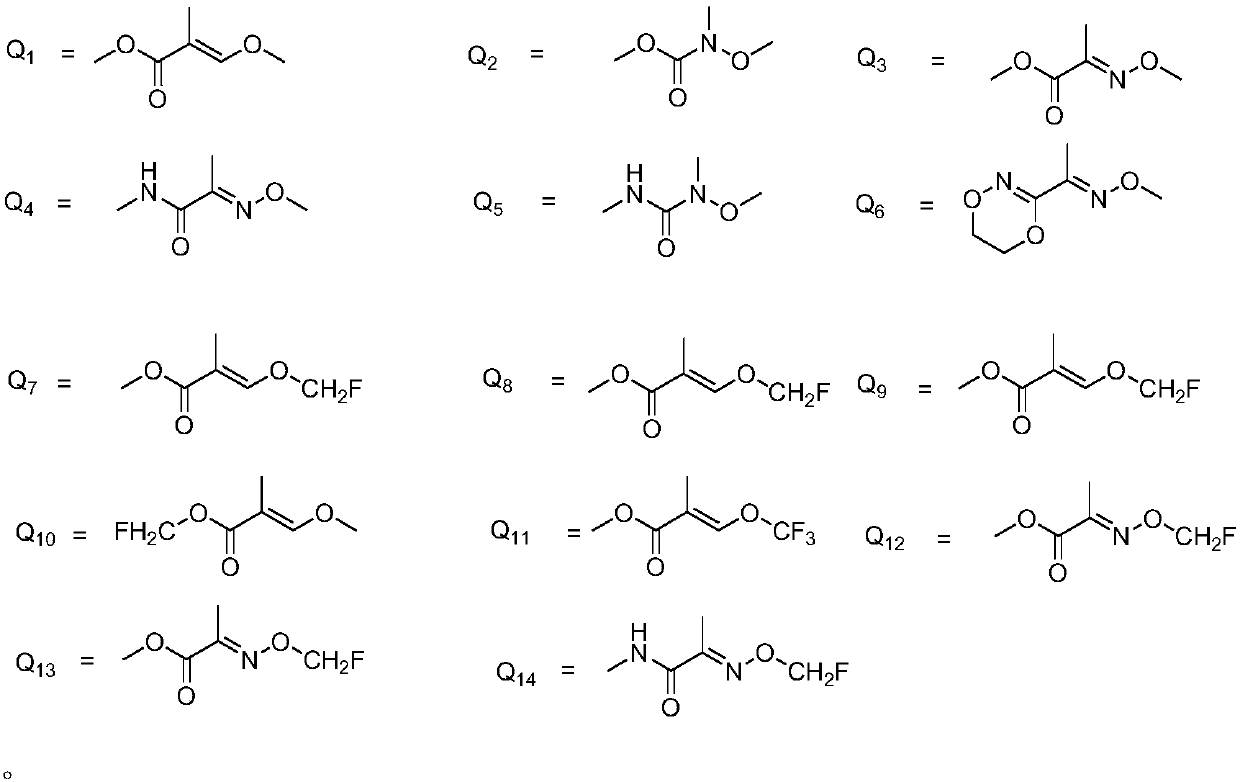
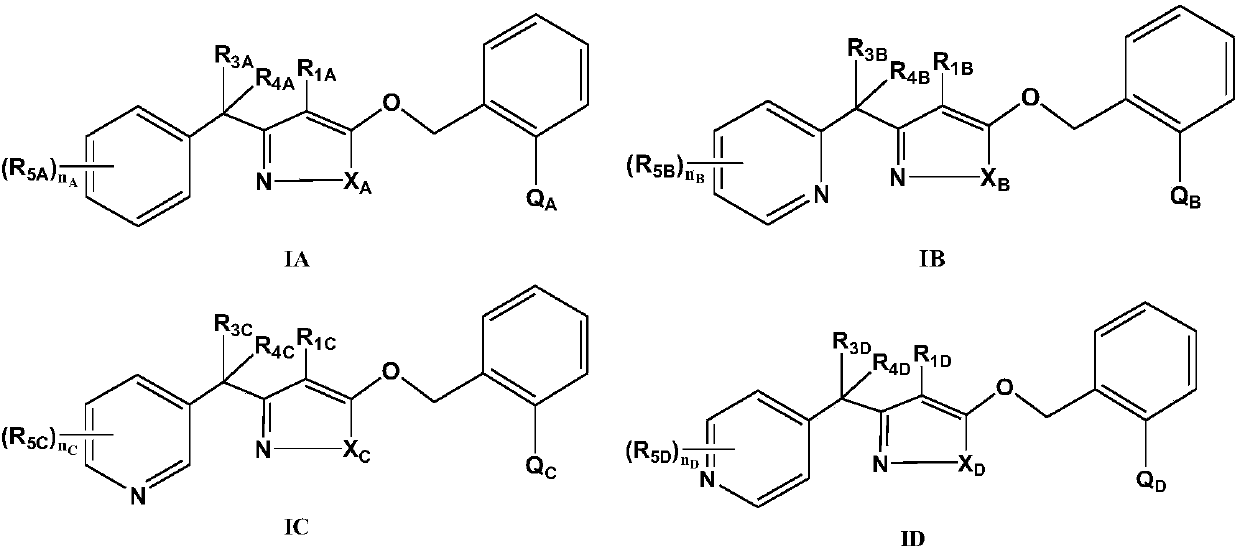
Substituted (hetero) arylmethylene pyrazole ether compound and preparation method and application thereof
A kind of methyl pyrazole ether and heteroaryl technology, applied in the field of substituted aryl methylene pyrazole ether compounds and their preparation, can solve the problems such as unsatisfactory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] Example 1: Synthesis of compound 111
[0164] Dissolve 1.3g (0.005mol) of 1a in 10ml of N,N-dimethylformamide, add 0.83g of potassium carbonate, stir for 0.5h, add 2a (1.26g) in batches, increase the temperature to 80℃, stir Reaction for 8h. After the reaction was detected by TLC, the reaction solution was poured into 50 ml of saturated brine, extracted three times with 100 ml of ethyl acetate, and dried. After desolvation, use petroleum ether: ethyl acetate 1000:1-500 as the eluent to obtain 1.86 g oily product by column chromatography.
[0165]
Embodiment 2
[0166] Example 2: Synthesis of compound 87
[0167] Dissolve 1.1g (0.005mol) 1b in 10ml of N,N-dimethylformamide, add 0.83g potassium carbonate, stir for 0.5h, add dropwise 2b (1.4g) DMF solution, and increase the temperature to 80℃ , Stir the reaction for 8h. After the reaction was detected by TLC, the reaction solution was poured into 50 ml of saturated brine, extracted three times with 100 ml of ethyl acetate, and dried. After desolvation, use petroleum ether: ethyl acetate 1000:1-200 as the eluent to obtain 1.68 g oily product by column chromatography.
[0168]
[0169] Other compounds of the present invention can be prepared with reference to the above examples.
[0170] NMR data of some compounds:
[0171] Compound 37: 1 HNMR (400MHz, DMSO) δ (ppm): 3.51 (s, 3H, CH 3 ), 3.60(s, 3H, CH 3 ),3.80(s,3H,CH 3 ),5.01(s,2H,CH 2 ), 5.94(s,1H,pyrazolyl1-H),7.13-7.53(m,9H,Ar-H), 7.67(s,1H,CH)
[0172] Compound 39: 1 HNMR (400MHz, DMSO) δ (ppm): 3.58 (s, 3H, CH 3 ),3.59(s,3H,CH 3 ),3.79(s,...
Embodiment 3
[0178] Example 3: 30% wettable powder
[0179]
[0180] The compound and other components are fully mixed and pulverized by an ultrafine pulverizer to obtain a 30% wettable powder product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com