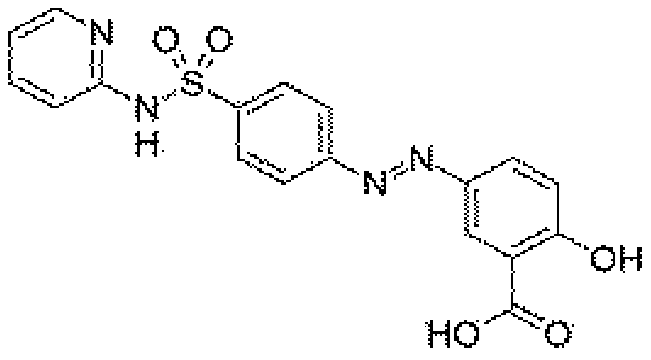
Ophthalmic composition containing sulfasalazine and hyaluronic acid
A technology for sulfasalazine and ophthalmic compositions, applied in the field of ophthalmic compositions, can solve the problems of increasing the process of forming salts, increasing the cost, complicating the preparation process, etc., and achieving the effects of improving solubility and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
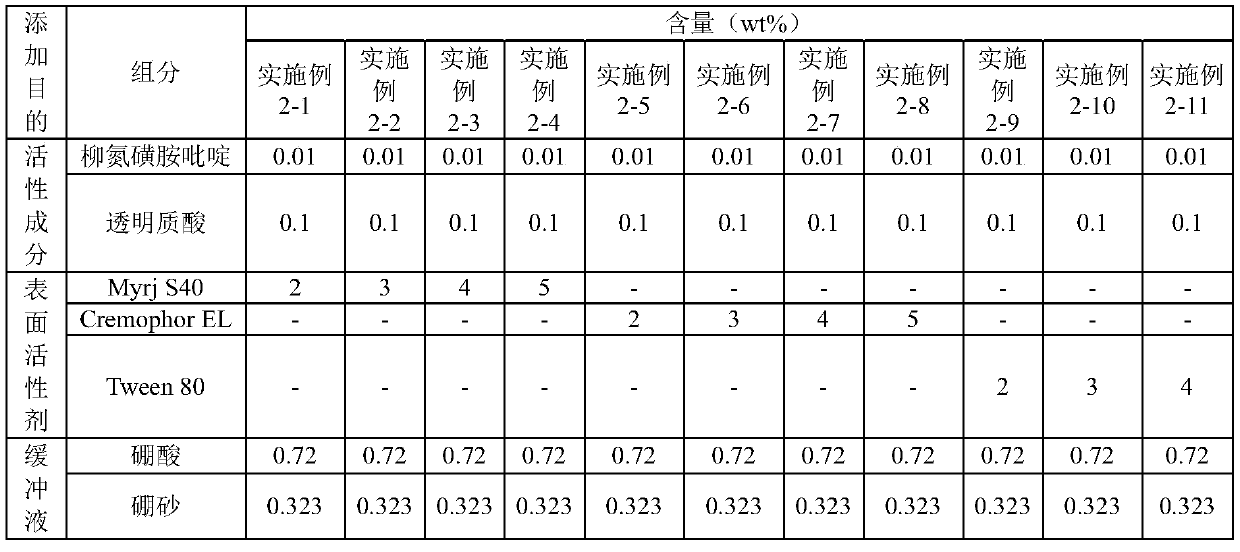
[0025] [Example 1] Preparation of composition in borax / boric acid buffer
[0026] A borax / boric acid buffer was used to prepare the compositions shown in Table 1 below, which contained Myrj S40, Cremophor EL or Tween 80 as surfactants.
[0027] [Table 1] Compositions prepared in borax / boric acid buffer
[0028]
Embodiment 2
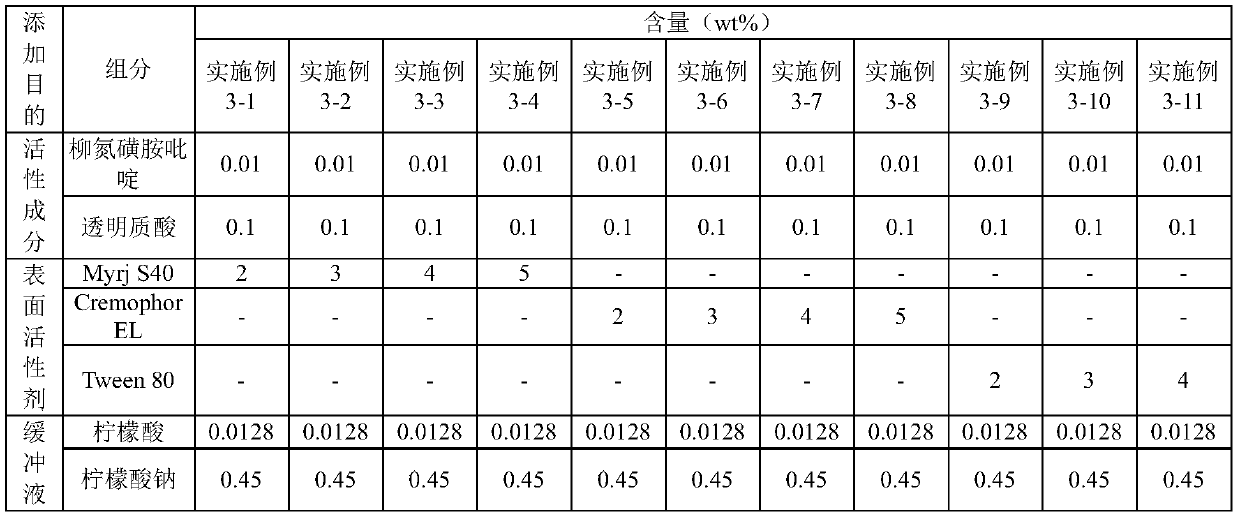
[0029] [Example 2] Preparation of composition in citrate buffer
[0030] The compositions shown in Table 2 below were prepared using citrate buffer containing Myrj S40, Cremophor EL or Tween 80 as surfactants.
[0031] [Table 2] Compositions prepared in citrate buffer
[0032]
Embodiment 3
[0033] [Example 3] Preparation of composition in trometamol (trometamol) buffer
[0034] The compositions shown in Table 3 below were prepared using trometamol buffer containing Myrj S40, CremophorEL or Tween 80 as surfactants.
[0035] [Table 3] Compositions prepared in tromethamine buffer
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com