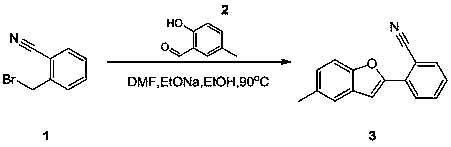
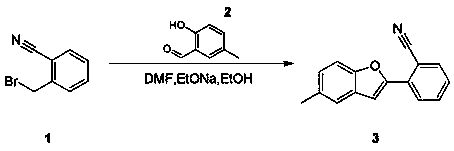
Synthesis method of 2-(5-methylbenzofuran-2-yl)benzonitrile
A synthesis method, the technology of benzonitrile, which is applied in the direction of organic chemistry, can solve the problems of no suitable industrial synthesis method, and achieve the effect of reasonable reaction process design, easy reaction and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Compound 2 (25.04 g, 184 mmol) was dissolved in N,N dimethylformamide (180 mL) and ethanol (90 mL), then sodium ethoxide (10.8 g, 199 mmol) and compound 1 (35 g , 179 mmol, ), stirred under nitrogen atmosphere, then reacted at 90°C for 3 hours, TLC (petroleum ether / ethyl acetate volume ratio = 5 / 1) showed that the reaction was complete. Then, concentrated to remove ethanol in the solvent. Add water (90 mL) to the mixture, and then filter to obtain the crude product. The crude product is subjected to column chromatography (gradient elution: petroleum ether ~ petroleum ether / ethyl acetate volume ratio = 100 / 1 ~ 20 / 1) to obtain the pure product Compound 3 (25 g, 107 mmol). Yield 60.3%.
[0013] 1 DMSO δ= 7.79~7.70 (m, 3H), 7.60~7.54 (m, 3H), 7.30~7.14 (m, 2 H), 2.34 (3, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com