Inert carrier Escherichia coli and its potential application
A technology of Escherichia coli and an inert carrier, applied to the inert carrier Escherichia coli and its potential application fields, can solve the problems of affecting the purification effect of epidemic diseases, and interfere with the results of detection and diagnosis, and achieve great application value and market prospects. The effect of specific bottlenecks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
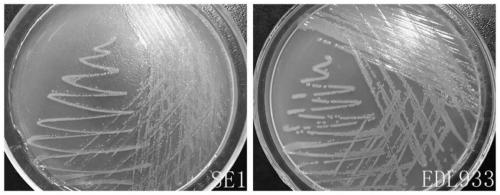
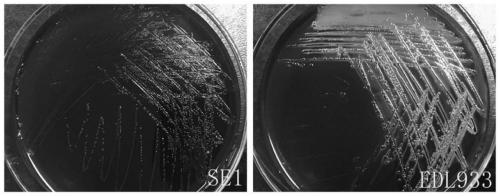
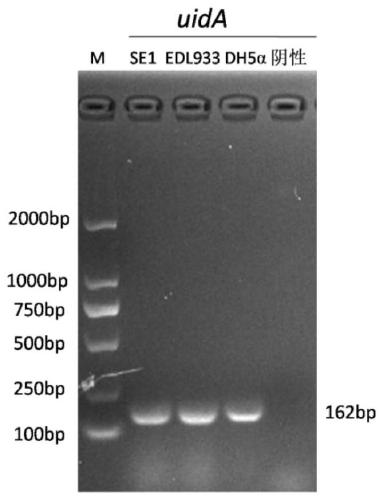
[0022] Example 1: Isolation and identification of Escherichia coli SE1
[0023] Since October 18, 2016, 360-day-old healthy laying hens were collected from the 2nd and 4th healthy chicken flocks of the first poultry farm of Mashan Poultry Farm in Wuxi, Jiangsu, and put them in an ultra-clean table, and cleaned them with alcohol. Disinfect the surface of the chicken body, aseptically remove the liver, spleen, intestinal tract and other organ tissues of the chicken, place them in a sterile petri dish, cut up the tissues and grind them at the same time, weigh them and suck the homogenate into a sterile test tube for later use. Buffered peptone water (BPW) was weighed and autoclaved for aliquots, and the collected tissue samples were added to BPW for overnight incubation at 37°C. Take 1 mL of Escherichia coli test broth (EC broth) for enrichment culture at 37°C, and after overnight culture, draw 20 μL of enrichment solution to streak culture on MacConkey agar medium at 37°C, pick ...
Embodiment 2
[0030] Example 2: Verification that inert Escherichia coli SE1 and chicken-derived serum do not produce non-specific agglutination
[0031] The inert carrier bacteria SE1 cultivated overnight were centrifuged at 4000 rpm for 10 min at 4°C, discarded the supernatant, resuspended with sterile PBS, centrifuged and washed three times, and then resuspended to different working concentration gradients of bacteria. Before the test, the bacterial solution was mixed with a vortex instrument, and the agglutination test was performed with sterile PBS and SPF chicken serum to ensure that the bacterial solution had no self-agglutination and no non-specific agglutination. In the ultra-clean bench (20-25°C), take several pieces of ordinary glass plates with a clean surface, resuspend and wash the carrier bacteria by centrifugation and washing for 3 times with PBS pre-cooled to 4°C, and then resuspend and dilute to the specified bacterial concentration. Use a micropipette to draw a drop of ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com