Bifidobacterium adolescentis ccfm1061, its fermented food and preparation method of bacterial agent
A bifidobacterium, fermented food technology, applied in bifidobacteria, bacteria used in food preparation, biochemical equipment and methods, etc., can solve liver function damage and edema, increased toxic and side effects of drugs, adverse reactions in the digestive tract, etc. problems, to achieve the effect of alleviating the toxicity of PFOA, reducing absorption and improving antioxidant capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Characteristics of Bifidobacterium adolescentis CCFM1061:
[0046] (1) Bacterial characteristics: Gram-positive bacteria that do not form spores and do not move;
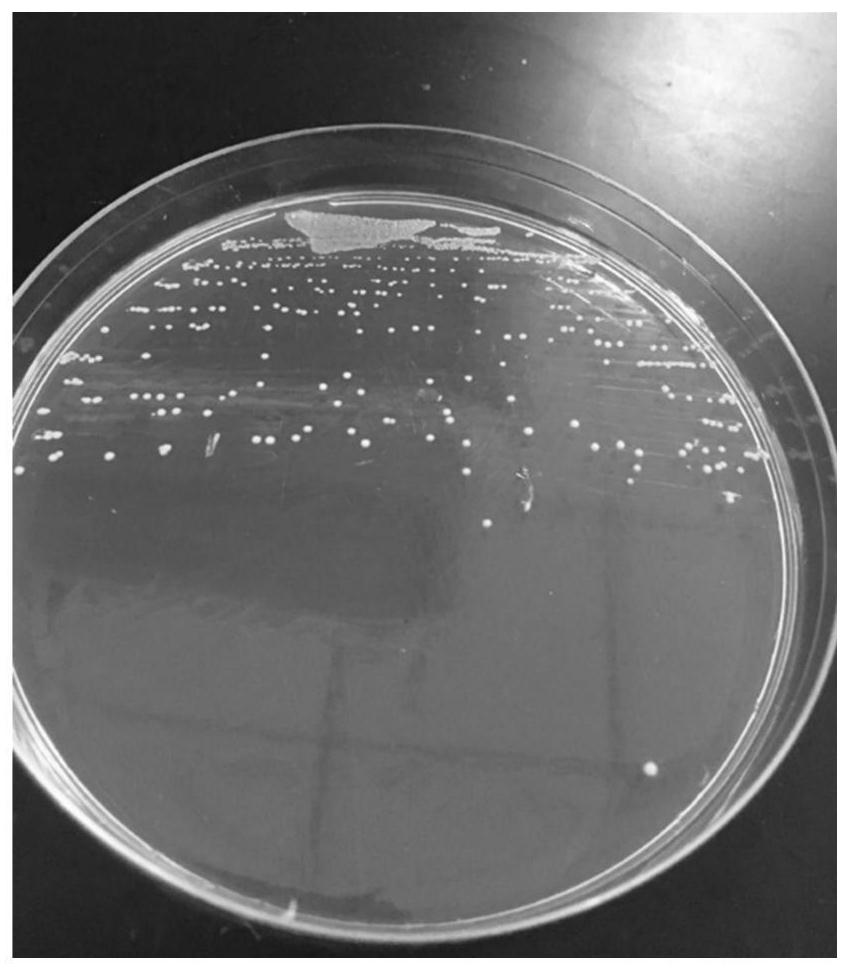
[0047] (2) Colony characteristics: After 36 hours of anaerobic culture, obvious colonies are formed, with a diameter between 0.5-1mm, a round front shape, a protruding side shape, neat edges, milky white, translucent, moist and smooth surface, and no pigmentation , see attached figure 1 ;
[0048] (3) Growth characteristics: under the condition of constant temperature and anaerobic at 37° C., cultivate in the modified mMRS medium for about 20 hours to reach the end of logarithmic phase.
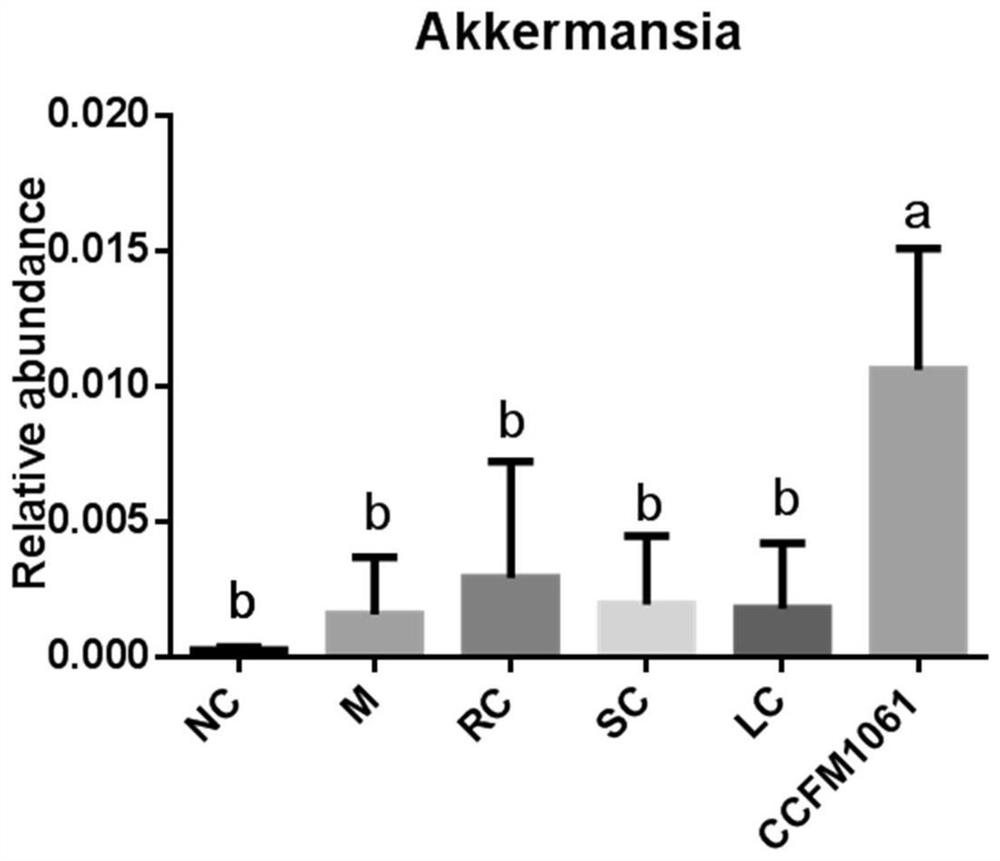
[0049] (4) Significantly increase the abundance of Akkermansia in the intestinal tract of NAFLD mice, and reduce the occurrence of diseases such as obesity, diabetic fatty liver and enteritis;
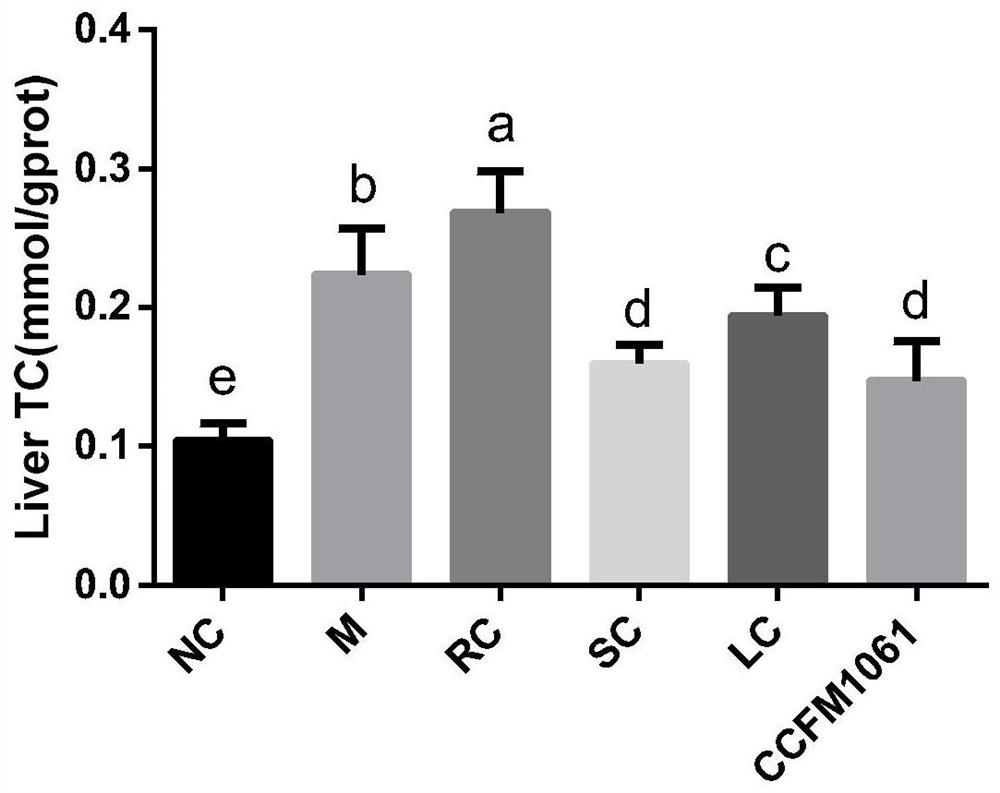
[0050] (5) Significantly improve lipid metabolism disorder in NAFLD mice;
[0051] (6) Significantly improved insulin resistanc...
Embodiment 2
[0093] Example 2: Bifidobacterium adolescentis CCFM1061 has no toxic side effects on C57BL / 6J mice
[0094] The Bifidobacterium adolescentis CCFM1061 bacteria were resuspended in 3% sucrose solution to make a concentration of 3.0×10 9CFU / mL bacterial suspension. Eight healthy male C57BL / 6J mice with a body weight of about 16-20 g were taken. After one week of adaptation to the environment, 0.2 mL of Bifidobacterium adolescentis CCFM1061 was administered orally once a day, observed for one week, and the death and body weight were recorded.
[0095] The results of these tests are listed in Table 1. These results indicate that daily feeding of 0.2 mL of 3.0×10 9 CFU / mL of Bifidobacterium adolescentis CCFM1061 had no significant effect on mice, no significant change in body weight, and no death. The appearance of the mice had no obvious pathological symptoms.
[0096] Table 1 Changes in body weight and death of mice
[0097]
[0098] Note: -: no death of mice
Embodiment 3
[0099] Example 3: Regulatory effect of Bifidobacterium adolescentis CCFM1061 on intestinal flora of NAFLD mice
[0100] Take 48 healthy male C57BL / 6J mice weighing 16-20g, adapt to the environment for 1 week, and randomly divide them into 6 groups: blank control group (NC), model control group (M), rosiglitazone control group (RC) , simvastatin control group (SC), Bifidobacterium adolescentis CCFM1061 intervention group (CCFM1061), Lactobacillus rhamnosus L10 intervention group (LC), each group contains 8 mice. The experimental animal groups and treatment methods are shown in Table 2:
[0101] Table 2 Grouping of experimental animals
[0102]
[0103]
[0104] At the end of the experiment, the fresh feces of the mice were collected and frozen at -80°C, the metagenome in the feces was extracted, and the structure of the intestinal flora was analyzed using a next-generation sequencer. At the end of the experiment, the mice were fasted for 12 hours, anesthetized by intrap...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com