Therapeutic RNA
A technology of identity and composition, applied in gene therapy, DNA preparation, recombinant DNA technology, etc., can solve problems such as difficult to treat and low long-term survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-
[0928] Example 1 - Materials and methods
[0929] B16F10 Tumor Model: Female C57BL / 6J mice (Jackson Laboratory; Bar Harbor, ME) aged 6 to 8 weeks and weighing between 17.0 and 20.9 g were acclimatized for at least three days prior to study participation. Mice had ad libitum access to chow (Harlan 2916 rodent chow, Massachusetts, USA) and sterile water, and were housed on a 12-hour light / dark cycle at 22°C±2°C and 55%±15% relative humidity. B16F10 cells were obtained from the American Type Culture Collection (ATCC) (Manassas, Virginia USA) (catalogue number CRL-6475), and were supplemented with 10% heat-inactivated fetal bovine serum (HI FBS) (Life technologies, Cat. No. 10082-147) in Dulbecco's Modified Eagle's Medium (DMEM) (Life technologies, Cat. No. 11995) in 5% CO 2 , Culture at 37°C. Cells were harvested using 0.25% trypsin-EDTA (Life technologies, Cat. No. 25200-056), resuspended in Dulbecco's Phosphate Buffered Saline (DPBS) (Life technologies, Cat. No. 14190-144), a...
Embodiment 2
[0959] Example 2 - Combination of three mRNAs reduces tumor volume in vivo
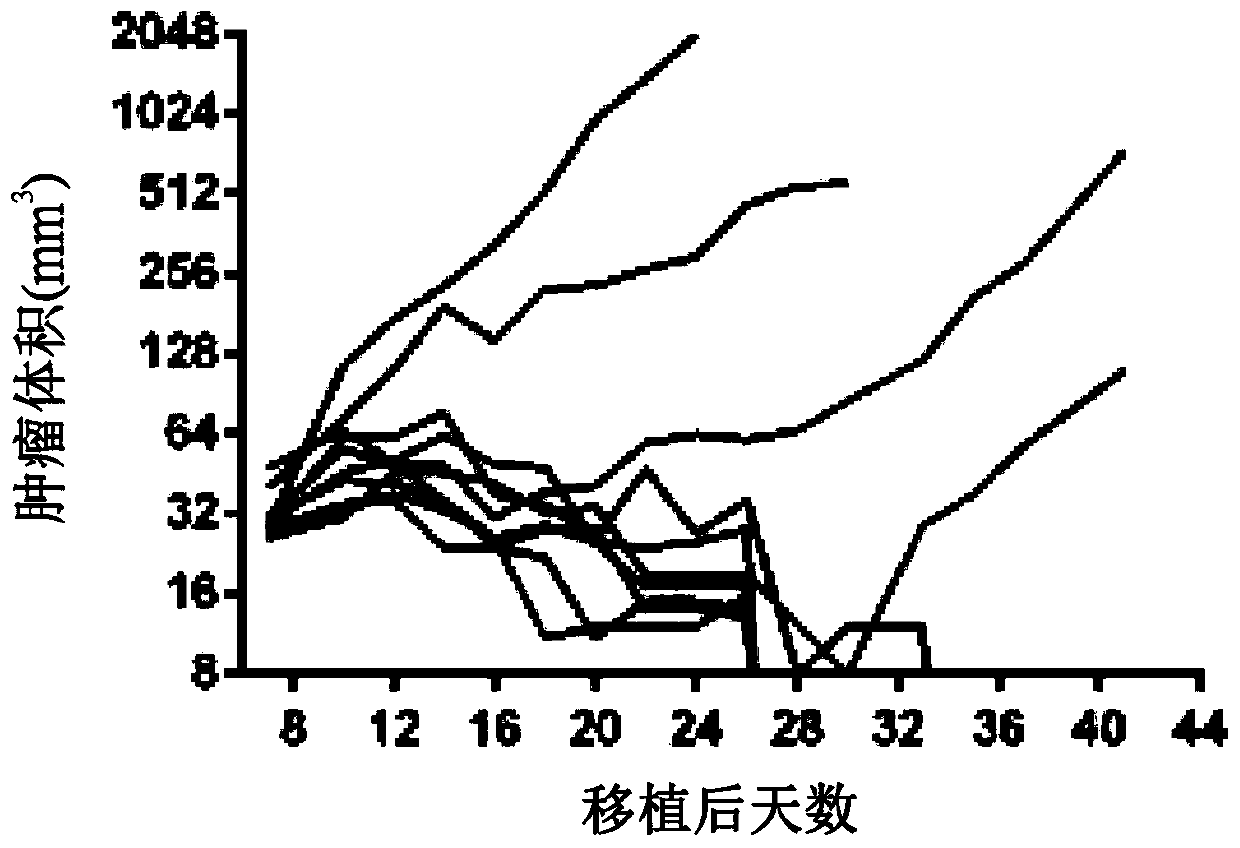
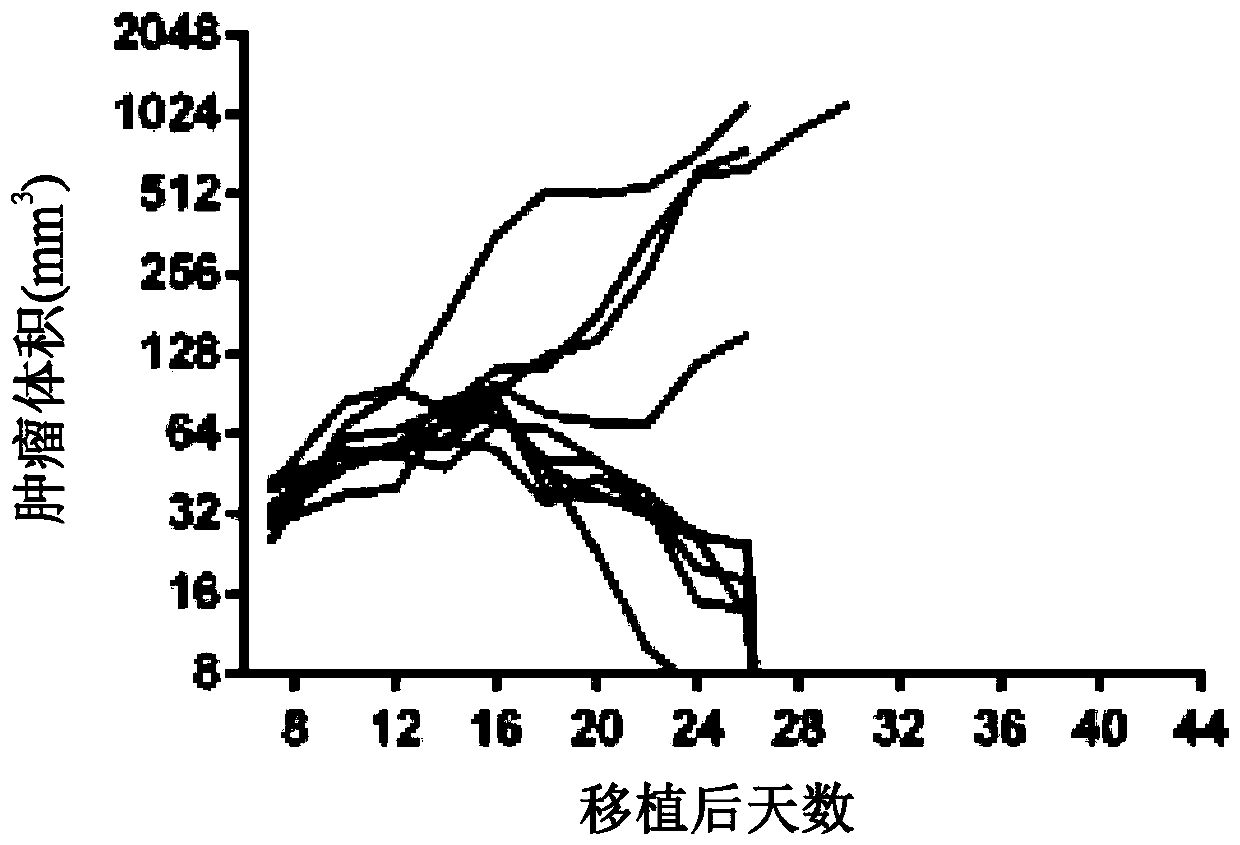
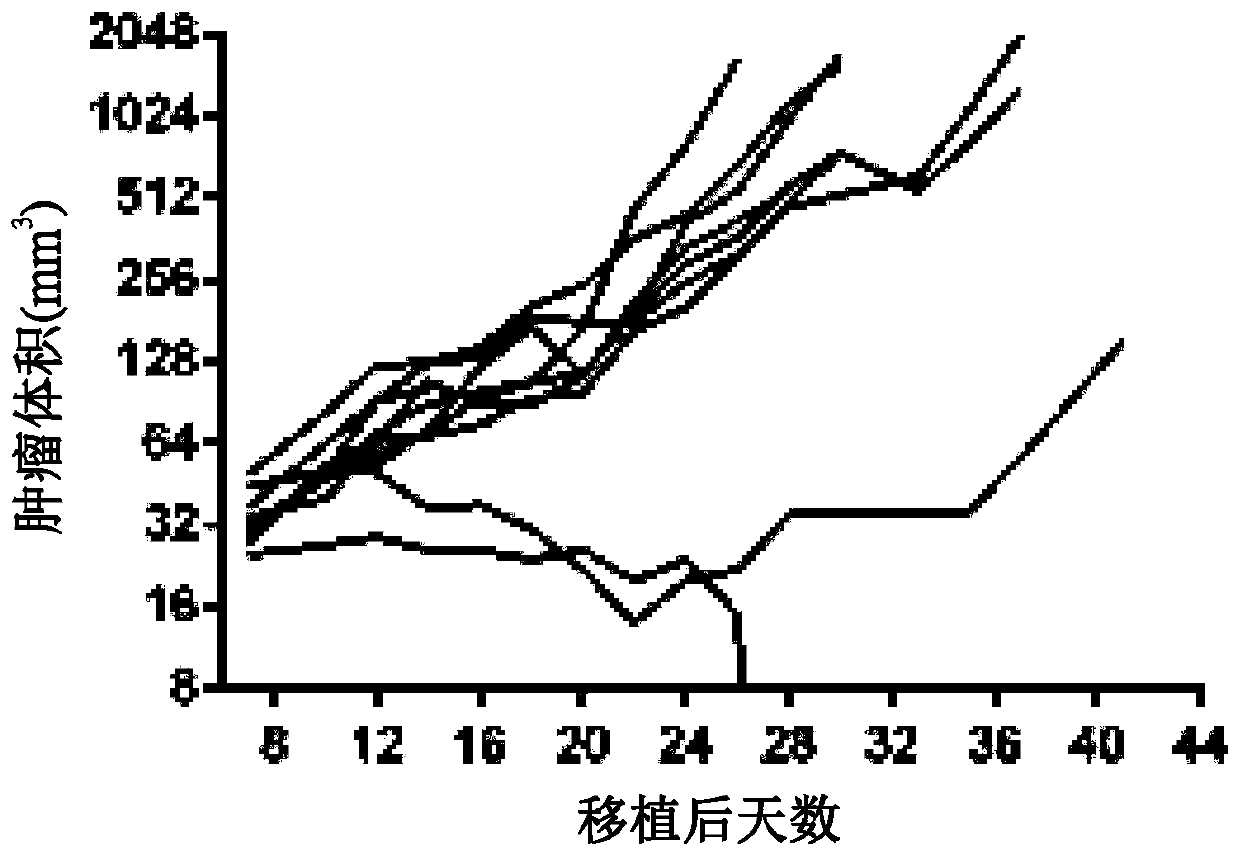
[0960] A mixture of modified mRNAs encoding GM-CSF, IL-2 and IL-12sc was injected into B16F10 tumor bearing mice and tumor growth was monitored until day 41. As shown in Figure 1, three mRNAs with ModA encoding GM-CSF, IL-2 and IL-12sc (SEQ ID NO: 32, 38 and 56; Figure 1A ), or three mRNAs with ModB encoding GM-CSF, IL-2 and IL-12sc (SEQ ID NOs: 35, 41 and 59; Figure 1B ), induced regression in 6 / 10 mice, whereas mice treated with control mRNA (ModA) encoding luciferase showed tumor regression in 1 / 10 animals ( Figure 1C ). These data were confirmed in a replicate study of a similar design ( Figure 1D to Figure 1G ). In repeated experiments, a mixture of cytokine mRNAs (GM-CSF, IL-2, IL-12sc) with ModA or ModB produced tumor regression in 5 / 9 mice, while treatment with control mRNAs in ModA and ModB, respectively 2 / 9 and 0 / 9 tumor regressions were consistently demonstrated.
[0961] The effe...
Embodiment 3
[0964] Example 3 - Combination of four mRNAs reduces tumor volume in vivo
[0965] Next, we tested the effect of adding a fourth mRNA to the cytokine mRNA mix. B16F10 tumor bearing mice received four intratumoral injections of the ModB cytokine mRNA cocktail encoding: i) GM-CSF, IL-2, IL-12sc (SEQ ID NOS: 59, 35 and 41; Figure 5A ), ii) GM-CSF, IL-15sushi, IL-12sc (SEQ ID NO:59, 53 and 41; Figure 5B ) and iii) GM-CSF, IL-15sushi, IL-12sc, IFNα (SEQ ID NOs: 59, 53, 41 and 47; Figure 5C ); and tumor growth was monitored until day 45. Each of the cytokine mRNA mixtures has an antitumor effect after intratumoral injection of GM-CSF, IL-2 and IL-12sc or the cytokine mRNA mixture of GM-CSF, IL-15sushi and IL-12sc, of which 4 / 8 tumors regressed; and 7 / 8 tumors regressed after treatment with GM-CSF, IL-15sushi, IL-12sc and IFNα. Mice treated with control mRNA (ModB) did not exhibit tumor regression ( Figure 5D ).
[0966] The antitumor activity of GM-CSF, IL-2, IL-12sc and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com