Immunotherapeutic tumor treatment method using an interleukin-2 receptor alpha, beta-selective agonist in combination with adoptive cell transfer therapy
An agonist, cell technology, used in drug combinations, antitumor drugs, chemical instruments and methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0120] It should be understood that the foregoing description and the following examples are intended to illustrate rather than limit the scope of the disclosure. Other aspects, advantages and modifications within the scope of the invention will be apparent to those skilled in the art to which this disclosure pertains.
[0121] Materials and Methods
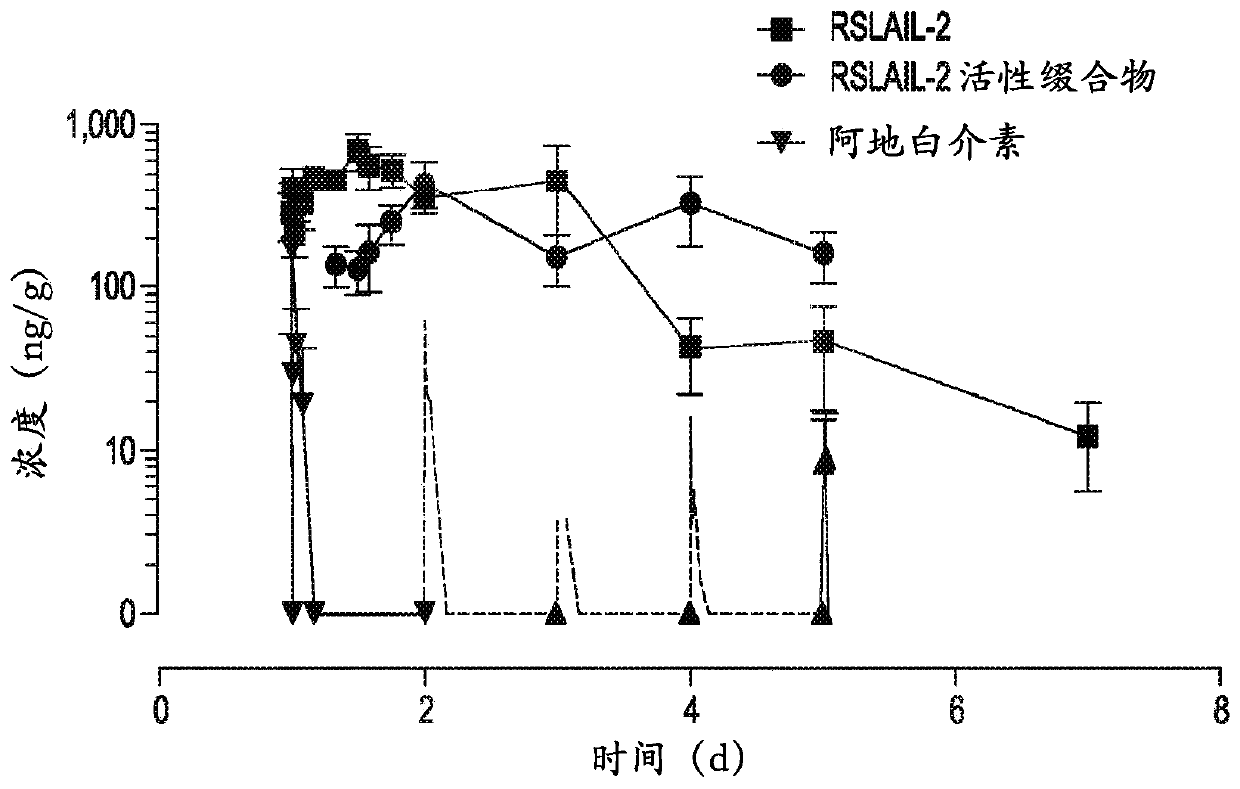
[0122] Recombinant human IL-2 having an amino acid sequence identical to that of aldesleukin was cloned and expressed and used to prepare an exemplary long-acting IL-2Rαβ-biased agonist referred to herein as RSLAIL-2.
example 1
[0125] PEGylation of rIL-2 with mPEG2-C2-Fmoc-20kD-NHS
[0126] 1.44 mg / ml of purified rIL-2 (106.4 mL) was charged to the first container, followed by the addition of 53.6 mL of formulation buffer (10 mM sodium acetate, pH 4.5, 5% trehalose). The measured pH was 4.62 and the measured temperature was 21.2°C. The PEG reagent C2-PEG2-FMOC-NHS-20K (obtained as described in WO 2006 / 138572) (13.1 g) was charged into a second container, followed by the addition of 73.3 mL of 2 mM HCl. The resulting solution was manually vortexed for 25 minutes. Sodium borate (0.5M, pH 9.8) was added to the first vessel to raise the pH to approximately 9.1, followed by the contents of the second vessel containing the PEG reagent being added to the second vessel over a period of 1 to 2 minutes. in a container. A rinse step was then performed by filling 8.1 mL of 2 mM HCl into the second container and adding the contents to the first container. For the conjugation reaction, the final rIL-2 concentr...
example 2
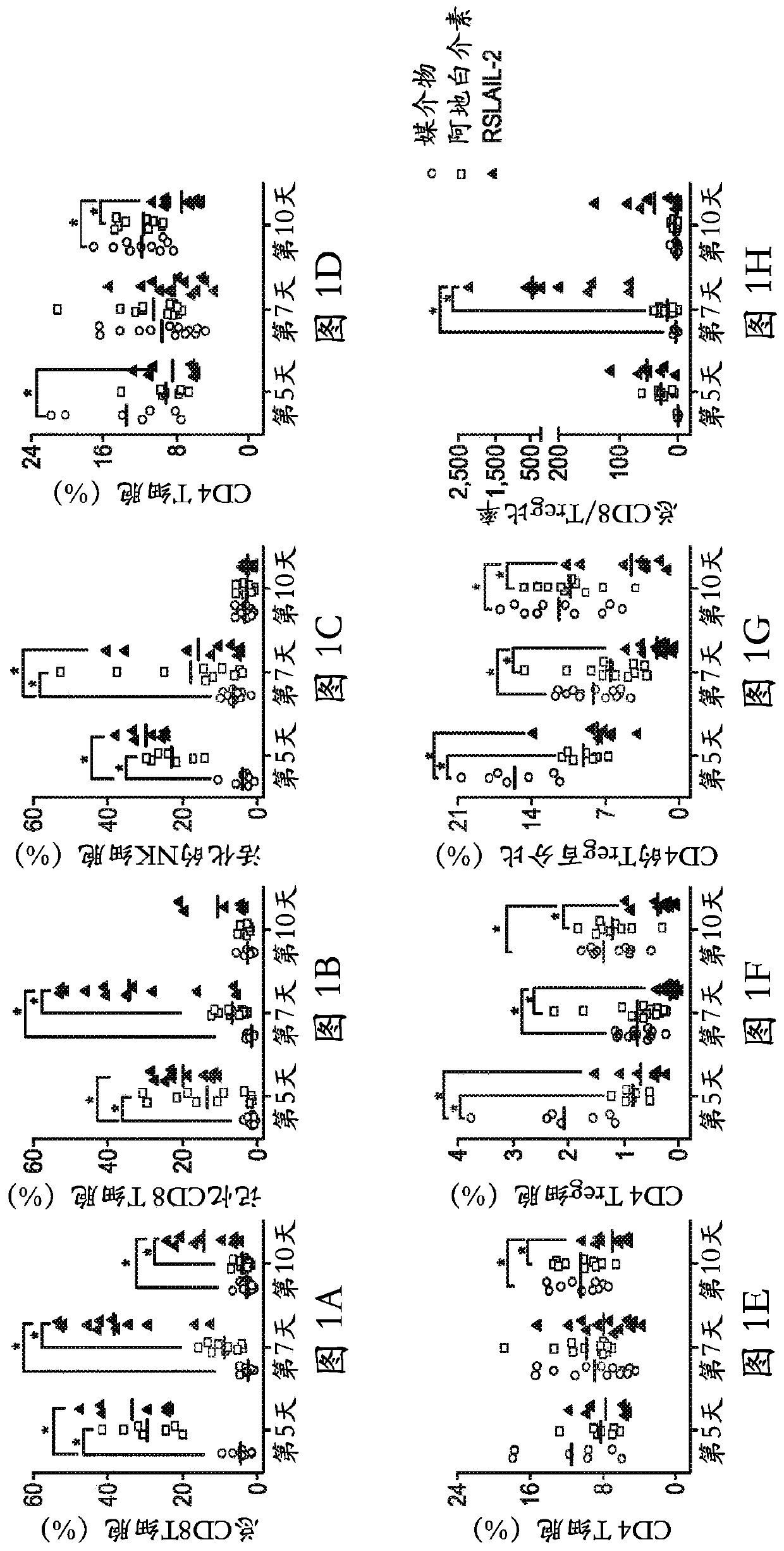
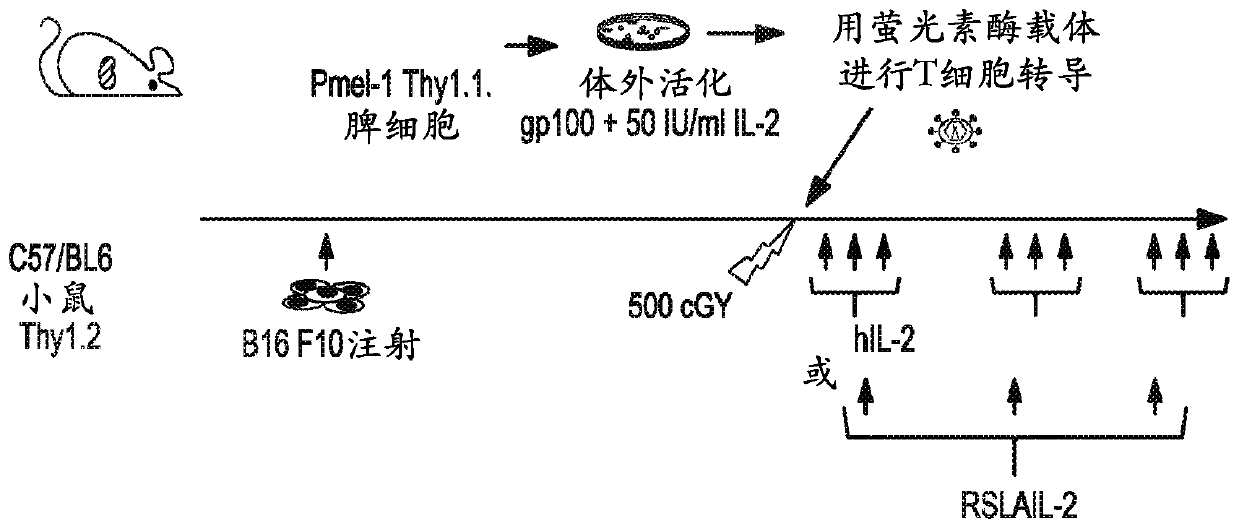
[0128] Receptor bias and associated immunotherapeutic properties of RSLAIL-2
[0129] Binding affinity to IL-2 receptor and receptor bias in relation to immunostimulatory profile: The binding affinity of RSLAIL-2 to IL-2Rα and IL-2Rβ was directly measured by surface plasmon resonance (Biacore T-100) and compared to It was compared with the binding affinity of clinically available IL-2 (aldesleukin). Anti-human antibodies (Invitrogen) were coupled to the surface of the CM-5 sensor chip using EDC / NHS chemistry. Then, human IL-2Rα-Fc or IL-2Rβ-Fc fusion proteins were used as ligands captured on this surface. Serial dilutions of RSLAIL-2 and its active IL-2 conjugate metabolites (1-PEG- and 2-PEG-IL-2) were prepared in acetate buffer, pH 4.5, starting at 5 mM. These dilutions were allowed to bind to the ligand for 5 minutes, and concentration was plotted against response units (RU) bound to determine EC50 values. The affinity of each isotype for each IL-2 receptor subtype was c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com