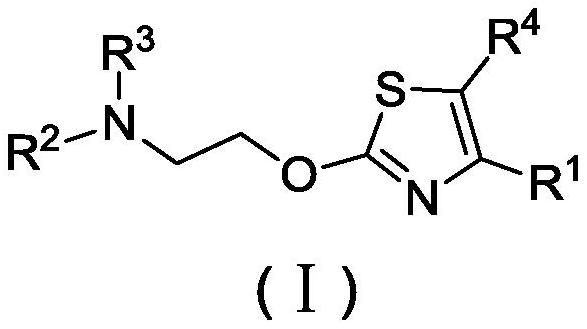
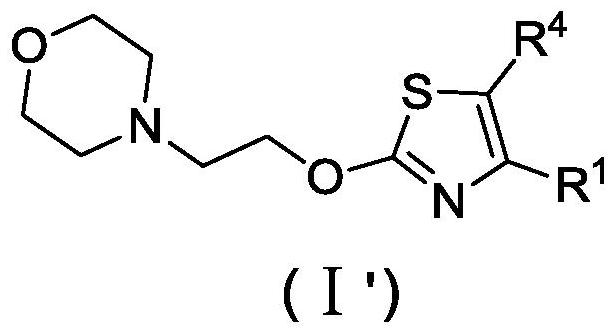
Novel thiazole derivative or salt, isomer, preparation method and application thereof
A technology of tautomers and stereoisomers, which is applied in the therapeutic field to achieve the effects of fast absorption, low clearance rate and long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
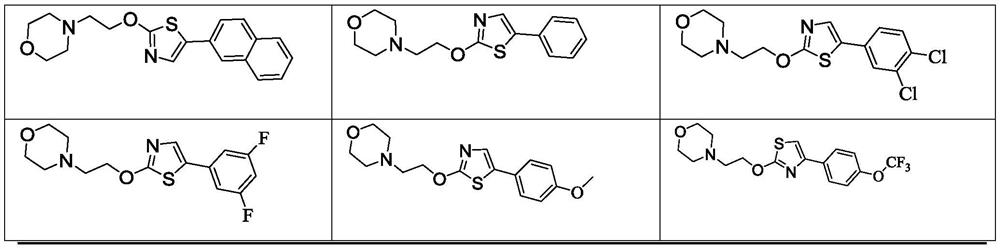
[0059] Example 1: Preparation of 4-{2-{[5-(naphthalene-2-yl)thiazol-2-yl]oxy}ethyl}morpholine
[0060]
[0061] Step 1, at room temperature, compound IIa (2.43g, 10mmol), compound VI (1.97g, 15mmol), Cs 2 CO 3 (9.75g, 30mmol) was added into DMF (30mL), heated to 100°C, reacted for 6h, and the raw materials were completely reacted. The reaction solution was cooled to room temperature, and H 2 O (30mL), extracted three times with ethyl acetate (50mL*3), the organic phase was washed with water and saturated brine successively, the organic phase was dried with anhydrous sodium sulfate, filtered, the filtrate was spin-dried, and the crude product was used as eluent petroleum ether: Ethyl acetate=3:1 was passed through the column, spin-dried, and vacuum-dried to obtain 2.50 g of the product. Yield: 85.2%.
[0062] Step 2, at room temperature, compound Va (2.50g, 8.5mmol), compound naphthalene-2-ylboronic acid (1.76g, 10.2mmol), CsF (1.55g, 10.2mmol) were added to 1,4-dioxane / ...
Embodiment 2
[0065] Example 2: Preparation of 4-{2-{[5-phenylthiazol-2-yl]oxy}ethyl}morpholine
[0066]
[0067] According to the preparation method of Example 1, the 2-naphthylboronic acid in step 2 was replaced by an equimolar amount of phenylboronic acid to obtain the title compound with a yield of 80.4% and a purity of 99.46%.
[0068] ESI-MS:m / z=291.2(M+H) + .
[0069] 1 H NMR (400MHz, CDCl 3 )δ7.46–7.42(m,2H),7.39–7.34(m,2H),7.31–7.27(m,2H),4.57(t,J=5.6Hz,2H),3.76–3.73(m,4H) , 2.83 (t, J=5.6Hz, 2H), 2.61–2.54 (m, 4H).
Embodiment 3
[0070] Example 3: Preparation of 4-{2-{[5-(3,4-dichlorophenyl)thiazol-2-yl]oxy}ethyl}morpholine
[0071]
[0072] According to the preparation method of Example 1, the 2-naphthylboronic acid in step 2 was replaced by an equimolar amount of 3,4-dichlorophenylboronic acid to obtain the title compound with a yield of 82.5% and a purity of 99.77%.
[0073] ESI-MS:m / z=359.2(M+H) + .
[0074] 1 H NMR (400MHz, CDCl 3 )δ7.51(d, J=2.1Hz, 1H), 7.42(d, J=8.4Hz, 1H), 7.30(s, 1H), 7.24(d, J=2.1Hz, 1H), 4.58(t, J=5.6Hz, 2H), 3.76–3.72(m, 4H), 2.82(t, J=5.6Hz, 2H), 2.61–2.53(m, 4H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com