Method for preparing btk inhibitor
A solvent and reaction mixture technology, applied in the field of preparation of BTK inhibitors, can solve the problem of low total yield of starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
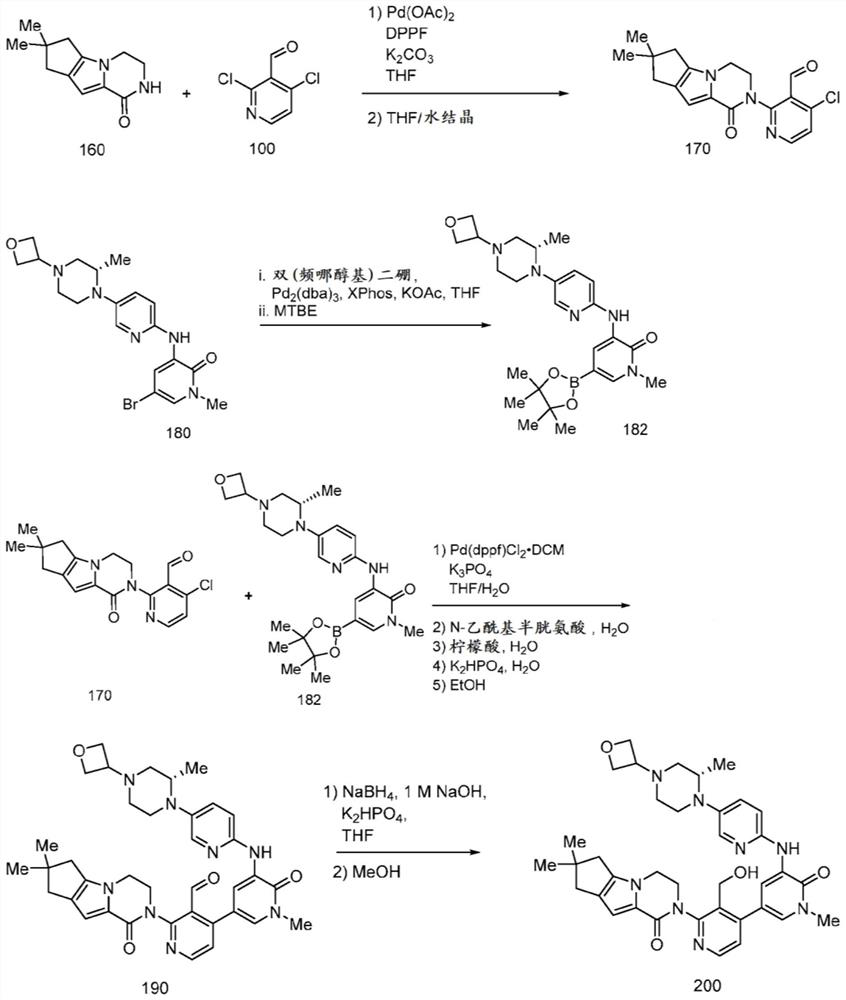
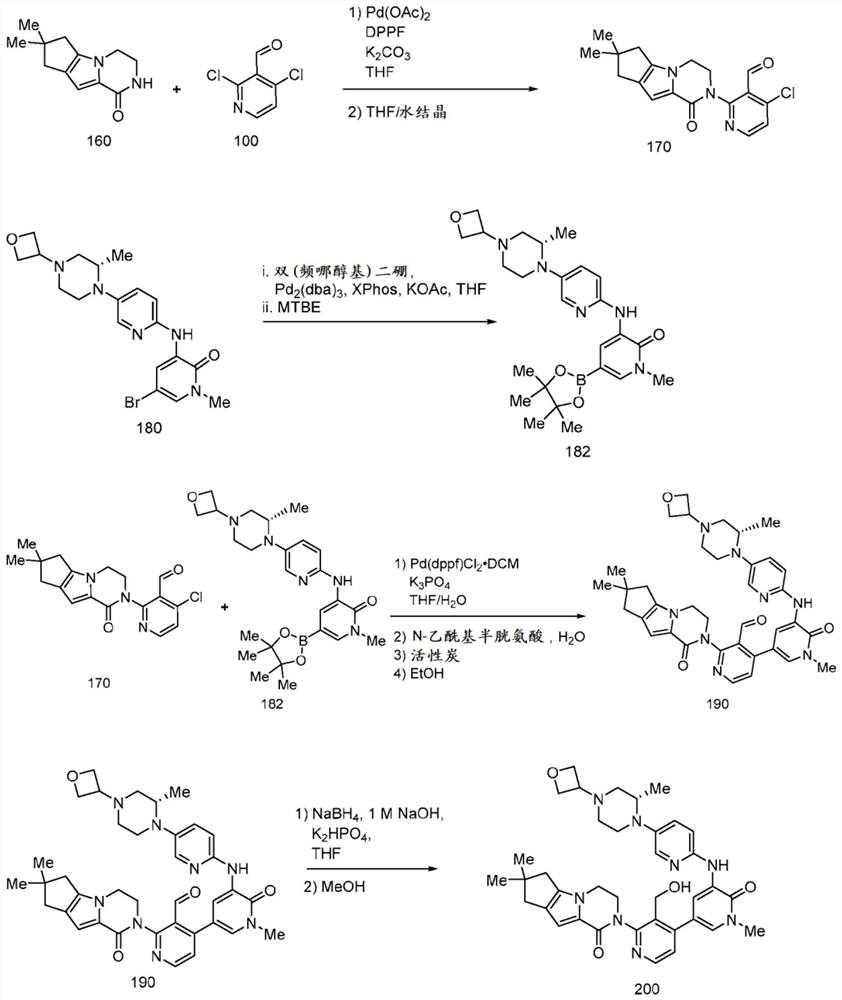
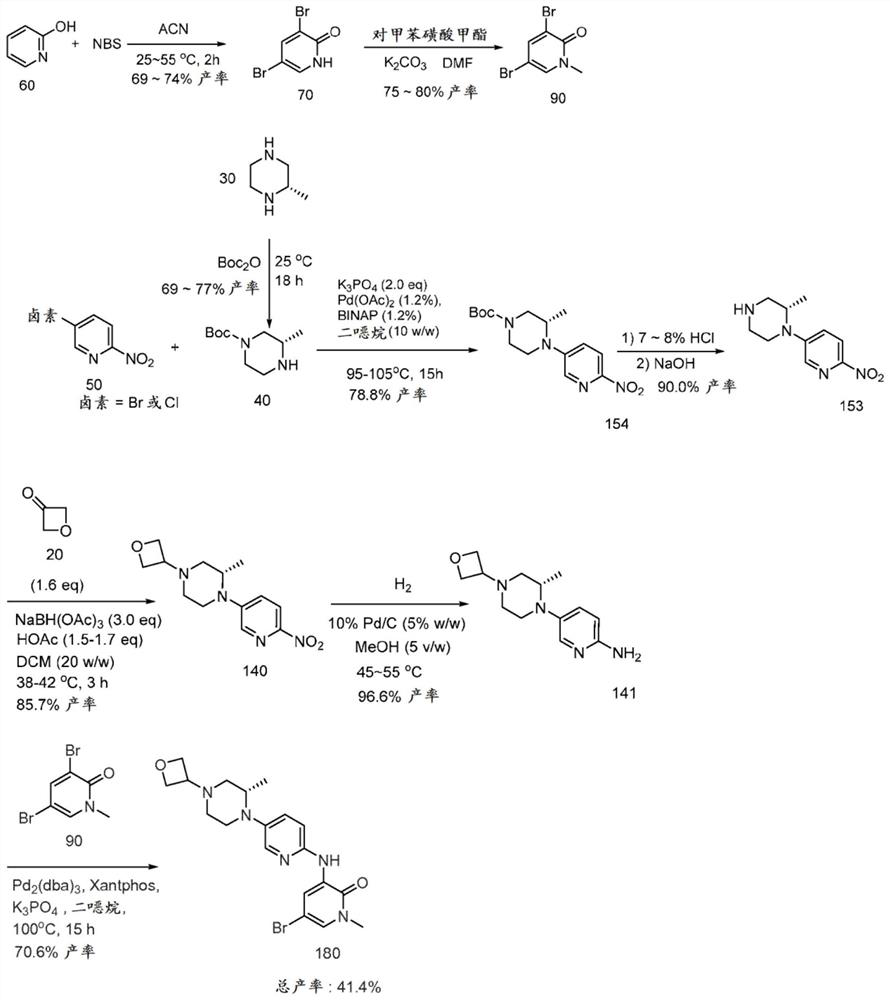
[0147] Preparation of compound 400
[0148] In some aspects of the invention, tricyclic lactam compound 400, its stereoisomers, its geometric isomers, its tautomers, and its salts can be prepared from compounds 300 and 310 according to the following reaction schemes:
[0149]
[0150] The method of preparing compound 400 includes forming a reaction mixture comprising an organic solvent, an organic base, and compounds 300 and 310, and reacting the reaction mixture to form a reaction product mixture comprising the tricyclic lactam of compound 400.
[0151] R 1a , R 1b , R 2a , R 2b , R 3a , R 3b , R 4a and R 4b independently selected from H and C 1-6 alkyl. R 5 selected from H, C 1-6 Alkyl, cycloalkyl, aryl, substituted aryl, benzyl, substituted benzyl, heteroaryl, substituted heteroaryl. In some respects, R 1a , R 1b , R 3a , R 3b , R 4a , R 4b and R 5 is H, and R 2a and R 2b yes-ch 3 .
[0152] Halogen is as described elsewhere herein. In some aspect...
Embodiment
[0224] The figures and examples provide exemplary methods for preparing the disclosed compounds; those skilled in the art will appreciate that other synthetic routes may be used to synthesize the compounds. Although particular starting materials and reagents are described and discussed in the Figures and Examples, other starting materials and reagents can be substituted to provide various derivatives and / or reaction conditions. In addition, many of the described and exemplary methods can be further modified in light of the present disclosure using conventional chemical methods well known to those skilled in the art.
[0225] In the examples, equivalent weights and equivalent ratios are based on reference starting materials for each reaction. Volume per weight value, eg, L / kg and mL / g, refers to the volume of liquid components based on the weight of the reference starting material for each reaction.
[0226] Analytical method
[0227] High pressure liquid chromatography (HPLC...
Embodiment 5 to 8
[0229] Examples 5 to 8. Column: Waters Atlantis T3 (4.6*150mm 3μm). Mobile phase A: 10 mM ammonium formate pH 3.7. Mobile phase B: CH 3 EN. Flow rate: 1.0 mL / min. Injection volume: 2.0uL. Column temperature: 45°C. UV detection wavelength: 315nm. Thinner: CAN.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com