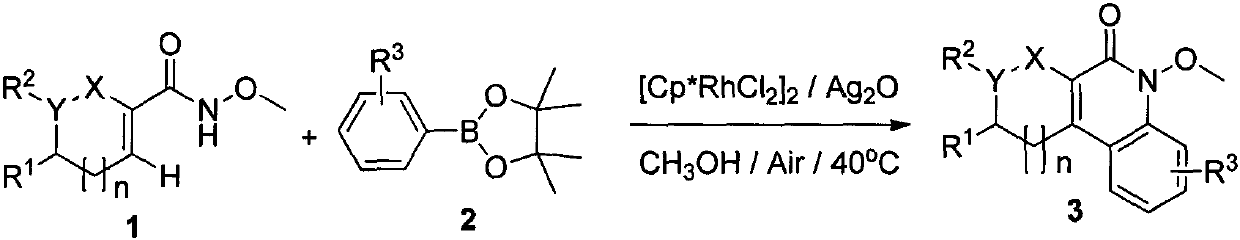
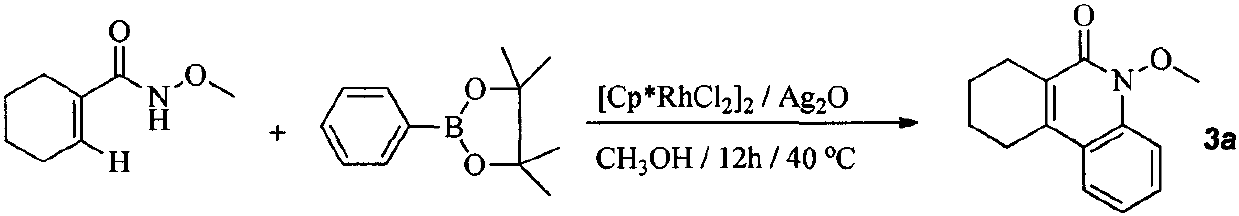Preparation method of 3,4-cycloalkylquinoline-2(1H)-one compound
A technology of ketone compounds and cycloalkylquinolines, which is applied in the field of preparation of 3,4-cycloalkylquinolin-2(1H)-one compounds, and can solve problems such as no specific records
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017]
[0018] At room temperature, N-methoxycyclohexene-1-carboxamide (10mmol), phenyl pinacol ester (20mmol), silver oxide (40mmol), rhodium catalyst (4mol%) were added successively in a pressure-resistant reaction tube and methanol (10 mL). The reaction mixture was then reacted at 80°C for 20 hours. Stop the reaction, concentrate under reduced pressure to obtain the crude product, and finally wash with a mixed eluent of petroleum ether and ethyl acetate, and perform flash column chromatography to obtain the target product 3a. Yield 89%. yellow liquid. 1 H NMR (400MHz, CDCl 3 )δ=7.71(d, J=8Hz, 1H), 7.58-7.63(m, 1H), 7.54(t, J=7.6Hz, 1H), 7.23-7,29(m, 1H), 4.08(s, 3H), 2.85 (s, 2H), 2.69 (s, 2H), 1.76-1.91 (m, 4H).
Embodiment 2
[0020]
[0021] At room temperature, add N-methoxycyclohexene-1-carboxamide (10mmol), p-methoxyphenyl pinacol ester (30mmol), silver oxide (30mmol), rhodium catalyst successively in the pressure-resistant reaction tube (4mol%) and methanol (10mL). The reaction mixture was then reacted at 70°C for 24 hours. Stop the reaction, concentrate under reduced pressure to obtain the crude product, and finally wash with a mixed eluent of petroleum ether and ethyl acetate, and perform flash column chromatography to obtain the target product 3c. Yield 84%. Yellow solid, m.p.109.7-111.2°C; 1 HNMR (400MHz, CDCl 3 )δ=7.60(d, J=7.2Hz, 1H), 7.06(d, J=2.4Hz, 1H), 6.84(dd, J=2.4Hz, J=2.8Hz, 1H), 4.08(s, 3H) , 3.92 (s, 3H), 2.77-2.85 (m, 2H), 2.60-2.70 (m, 2H), 1.75-1.90 (m, 4H).
Embodiment 3
[0023]
[0024] At room temperature, N-methoxycyclohexene-1-carboxamide (10mmol), p-fluorophenyl pinacol ester (35mmol), silver oxide (40mmol), rhodium catalyst (4mol) were added successively in the pressure-resistant reaction tube. %) and ethanol (10 mL). The reaction mixture was then reacted at 100°C for 24 hours. Stop the reaction, concentrate under reduced pressure to obtain the crude product, and finally wash with a mixed eluent of petroleum ether and ethyl acetate, and perform flash column chromatography to obtain the target product 3c. Yield 80%. yellow liquid. 1 H NMR (400MHz, CDCl 3 )δ=7.64-7.72(m, 1H), 7.24-7.32(m, 1H), 6.93-7.02(m, 1H), 4.08(s, 3H), 2.82(s, 2H), 2.67(s, 2H) , 1.76-1.90 (m, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com