Application of a l-threonine transaldolase in the synthesis of florfenicol chiral intermediates
A technology of threonine and transaldolase, applied in the fields of biotechnology and chemical engineering, can solve the problems of cumbersome production steps, large environmental pollution and high production cost, and achieve the effects of short reaction time, reduced production cost and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
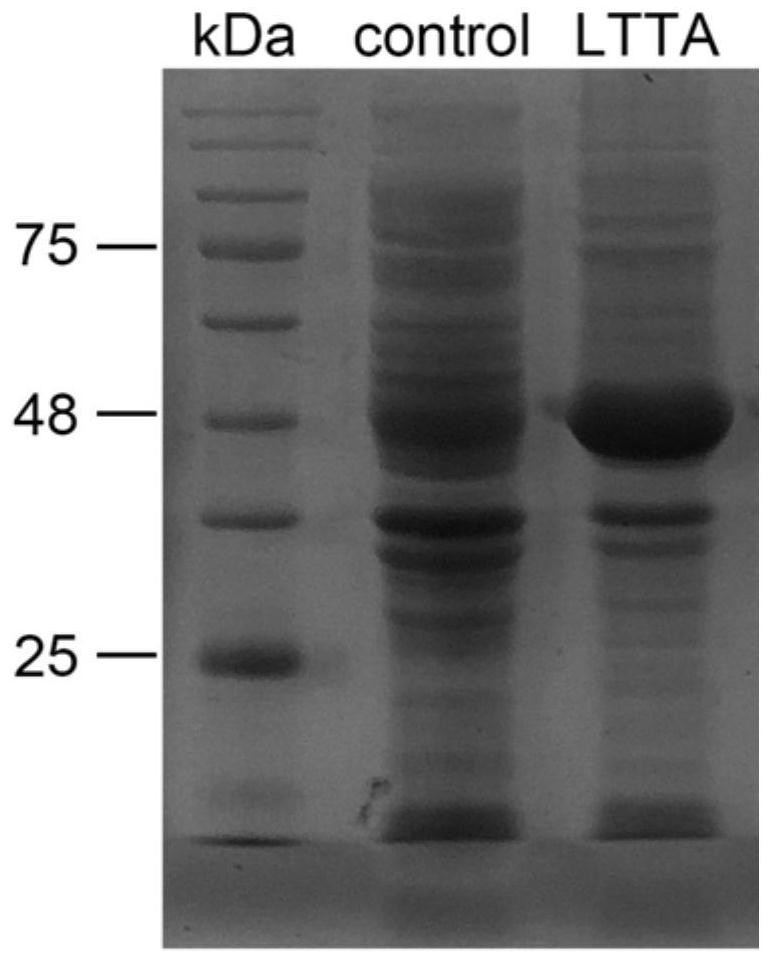
[0035] In this example, L-threonine aldolase derived from recombinant Escherichia coli was expressed by means of genetic engineering, and L-threonine aldolase was obtained; at the same time, whole cells were used as carriers to asymmetrically catalyze p-thiamphenicol Synthesis of (2S,3R)-p-thymphenylphenylserine from formaldehyde and L-threonine, by optimizing the reaction conditions, enantiomeric excess ee>99.9%, diastereomeric excess de=94.5%, substrate p-methylsulfone The conversion rate of phenylbenzaldehyde was 67.1%, and a set of L-threonine transaldolase was established to asymmetrically catalyze the synthesis of (2S,3R)-p-thiamphenylphenylserine from p-thiamphenylbenzaldehyde and L-threonine process conditions. The specific operation steps include:
[0036] (1) Obtain whole cells of L-threonine transaldolase by genetic engineering
[0037] A new L-threonine transaldolase gene (GenBankNo.CVTX01000156), SEQ ID NO: 2, was screened through gene mining, and the gene sequenc...
experiment example 1
[0060] The influence of experimental example 1 different metal ions (10mM) on LTTA catalytic activity
[0061] In order to explore the effect of different metal ions on the catalytic activity of LTTA, the following experiments were designed. The reaction system and conditions were the same as those in Example 1, except that the types of metal ions added were different, and the concentration of each metal ion was 10 mM. The experimental results are shown in Table 1 below. It can be seen that Mg, Cs, Li, Ca, and Ba can promote the catalytic activity of LTTA, and Mg has the best effect, and the enzymatic activity of LTTA has increased by 24%; Fe, Ni, Zn, and Cu can inhibit LTTA, and LTTA has different Under the condition of metal ions, the de value is 17%-81%.
[0062] Table 1 Effect of different metal ions on the activity of LTTA
[0063]
experiment example 2
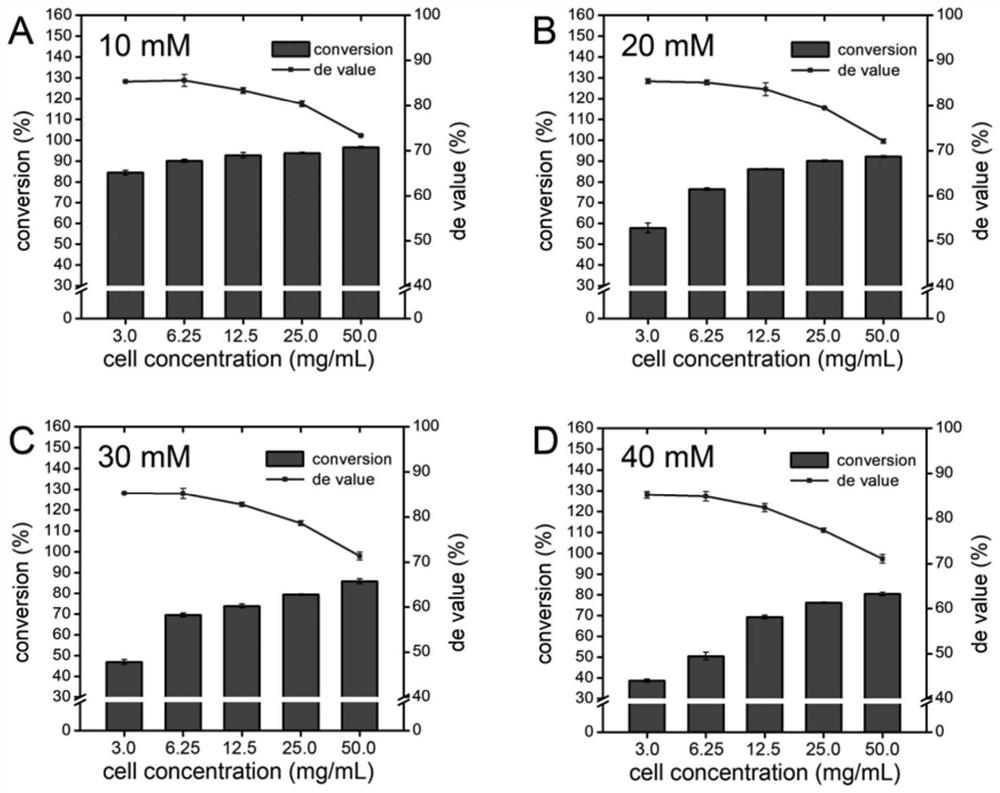
[0064] Experimental Example 2 Effects of Different Substrate Concentrations and Cell Concentrations on LTTA Conversion Rate and De Value
[0065] Taking p-thiamphenicyl benzaldehyde and L-threonine as substrates, the effects of different substrate p-thiamphenic benzaldehyde concentrations (10-40mM) and cell concentrations (3.0-50mg / mL) on LTTA conversion and de Value, the reaction system and conditions are the same as in Example 1, the difference is that the reaction temperature in this experimental example is 30 ° C, and the addition of organic solvent is 10% acetonitrile. The experimental results are attached image 3 shown. It can be seen that the de value of the product decreases with the increase of the cell concentration. When the cell concentration is 3.0 and 6.25mg / mL, the de value of the product does not change significantly (about 85%). When the cell concentration is greater than 6.25mg / mL , the de value of the product begins to decline (from 85% to 73%); when the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com