Heparan sulfate glycomimetic compounds and their pharmaceutical and cosmeceutical uses
一种化合物、酰基的技术,应用在硫酸化树状化合物领域,能够解决合成过程长等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 6
[0245] Example 6 shows that at least one compound of the present invention, compound 17c, is effective in the treatment of myeloma in mice, which indicates that this compound and structurally related compounds have therapeutic potential for myeloma and other potential cancers in humans.
[0246] Without being bound by theory, applicants hypothesize that the compounds of the invention may achieve an "aggregation effect" by utilizing multiple copies of sulfated sugar fragments in each dendrimer structure. This aggregation effect is advantageous because the repeating individual subunit structures can enhance the normally weaker interactions of the individual subunit structures in sulfated carbohydrates.
[0247] Cosmeceutical use
[0248] In addition to the aforementioned therapeutic uses, the compounds of the present invention are also believed to have potential cosmeceutical uses.
[0249] A reduction in fibrillar type I collagen is a hallmark of age-related or "unhealthy" ski...
Embodiment 11
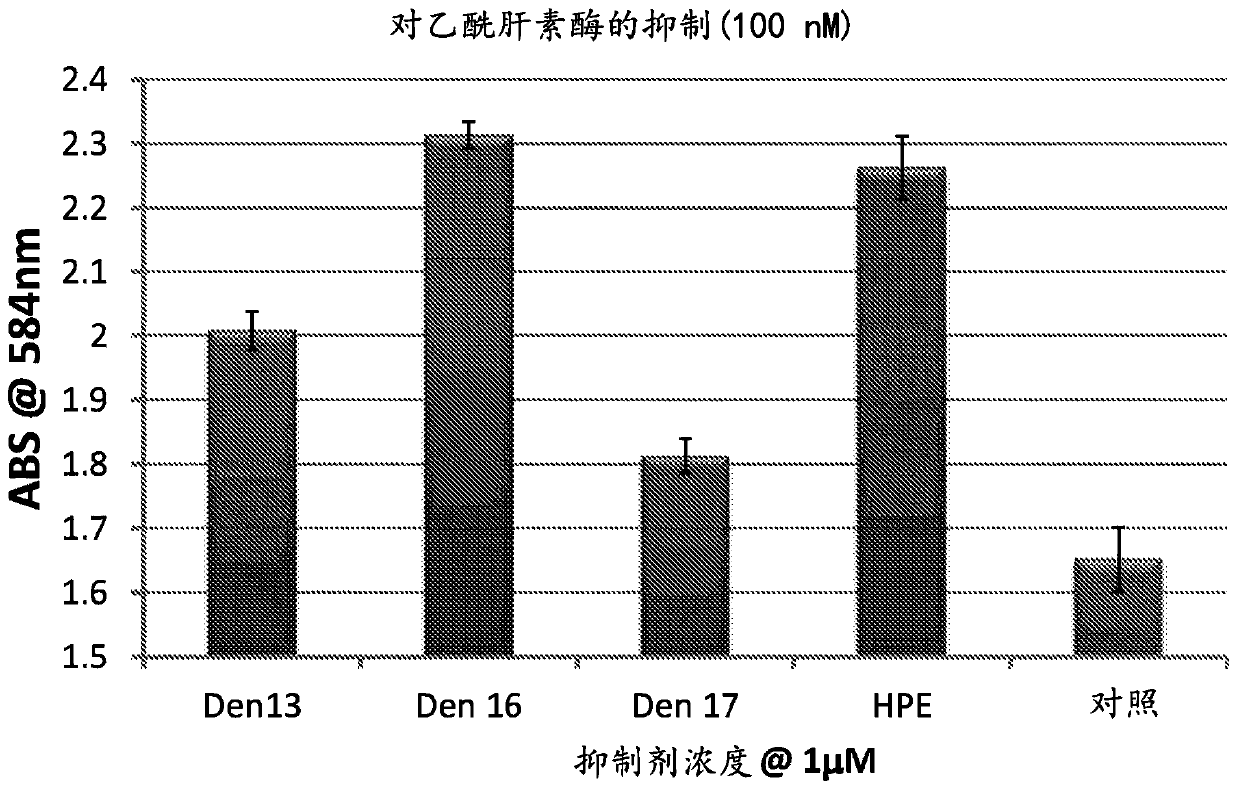
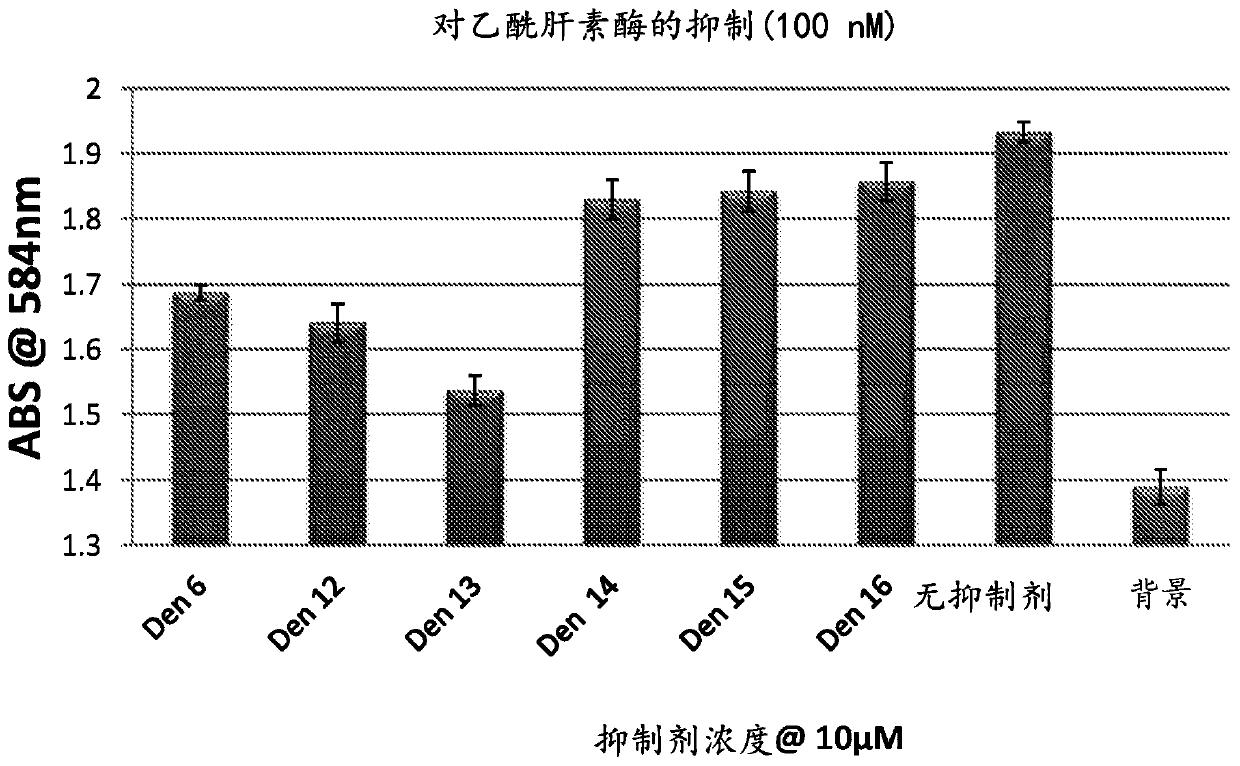
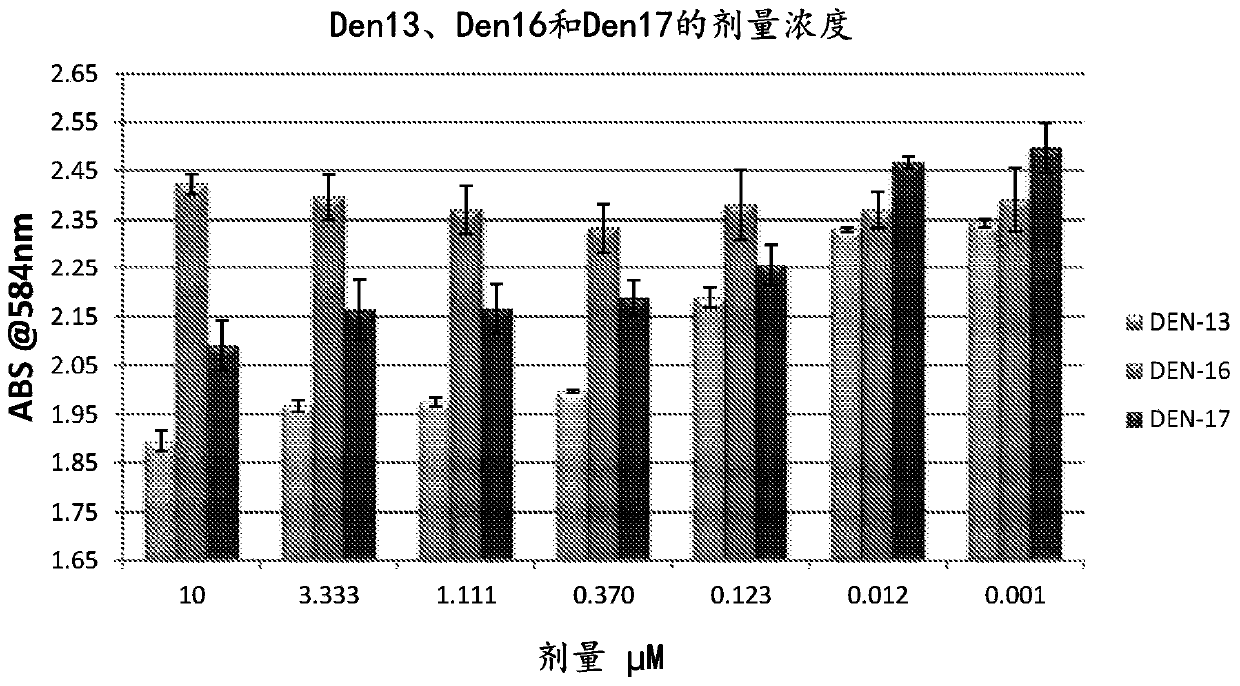
[0253] Example 11 shows that compounds 17c and 17d are also potent inhibitors of testicular hyaluronidase.
[0254] Formulation and Administration
[0255] The compounds of the present invention can be administered to patients by a variety of routes including orally, parenterally, by inhalation spray, topically, rectally, nasally, buccally or via implanted reservoirs. The compounds can also be administered by intracerebral, intracerebroventricular or intrathecal delivery. For parenteral administration, it can be intravenous, intraarterial, intramuscular or subcutaneous injection.
[0256] The dosage of a compound of the invention administered to a patient will vary widely depending on the condition of the patient and the nature and extent of the condition being treated. Usually, the dosage for an adult is about 0.01 μg / kg to about 1 g / kg, preferably about 0.01 mg / kg to about 100 mg / kg. The specific dosage required for any particular patient will depend on factors such as th...
Embodiment 1
[0305] Embodiment 1: the synthesis of tetrasuccinimide ester
[0306] The tetrasuccinimide ester was synthesized according to Scheme 7 below, which is described in WO 2014 / 084744.
[0307] Option 7
[0308]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com