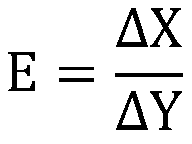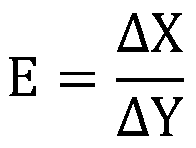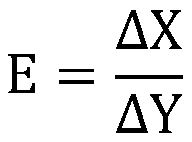Implant intervention medical instrument key parameter extraction and evaluation method based on axiom design
A technology of medical devices and design parameters, which is applied in the fields of instruments, calculations, electrical digital data processing, etc., and can solve problems such as increasing the complexity of implant medical device design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] This embodiment takes an intervertebral fusion device as an example to describe in detail the method for extracting and evaluating key parameters of implanted medical devices based on the axiom design of the present invention. The specific process of this method is as follows:
[0028] (1) Define user requirements according to the clinical use purpose of the implanted medical device and the anatomical constraints of the implanted position. For the intervertebral fusion device, since it is mainly used in anterior cervical decompression and fusion surgery, according to the clinical treatment effect and cervical anatomical structure characteristics of anterior cervical decompression and fusion surgery, the zero-notch vertebral fusion can be used. The user requirements for the design of the fusion cage are summarized as follows: a. Provide a stable mechanical environment for the surgical segment immediately after operation; b. Effectively relieve or eradicate the patient's p...
Embodiment 2
[0192] This embodiment takes the degradable vascular stent as an example to describe in detail the method for extracting and evaluating key parameters of an implanted medical device based on axiomatic design in the present invention. The specific process of this method is as follows:
[0193](1) Define user requirements according to the clinical use purpose of the implanted medical device and the anatomical constraints of the implanted position. For degradable vascular stents, the intervention site is the narrowed blood vessel, which mainly provides sufficient radial support for the blood vessel, and minimizes the rebound of the blood vessel in the early stage. When the blood vessel is remodeled, the stent can be gradually restored. Biodegradation, the final stent is completely degraded, and at the same time, the blood vessel achieves a new balance with the adjustment. Therefore, the user requirements for the design of degradable vascular stents can be summarized as follows: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com