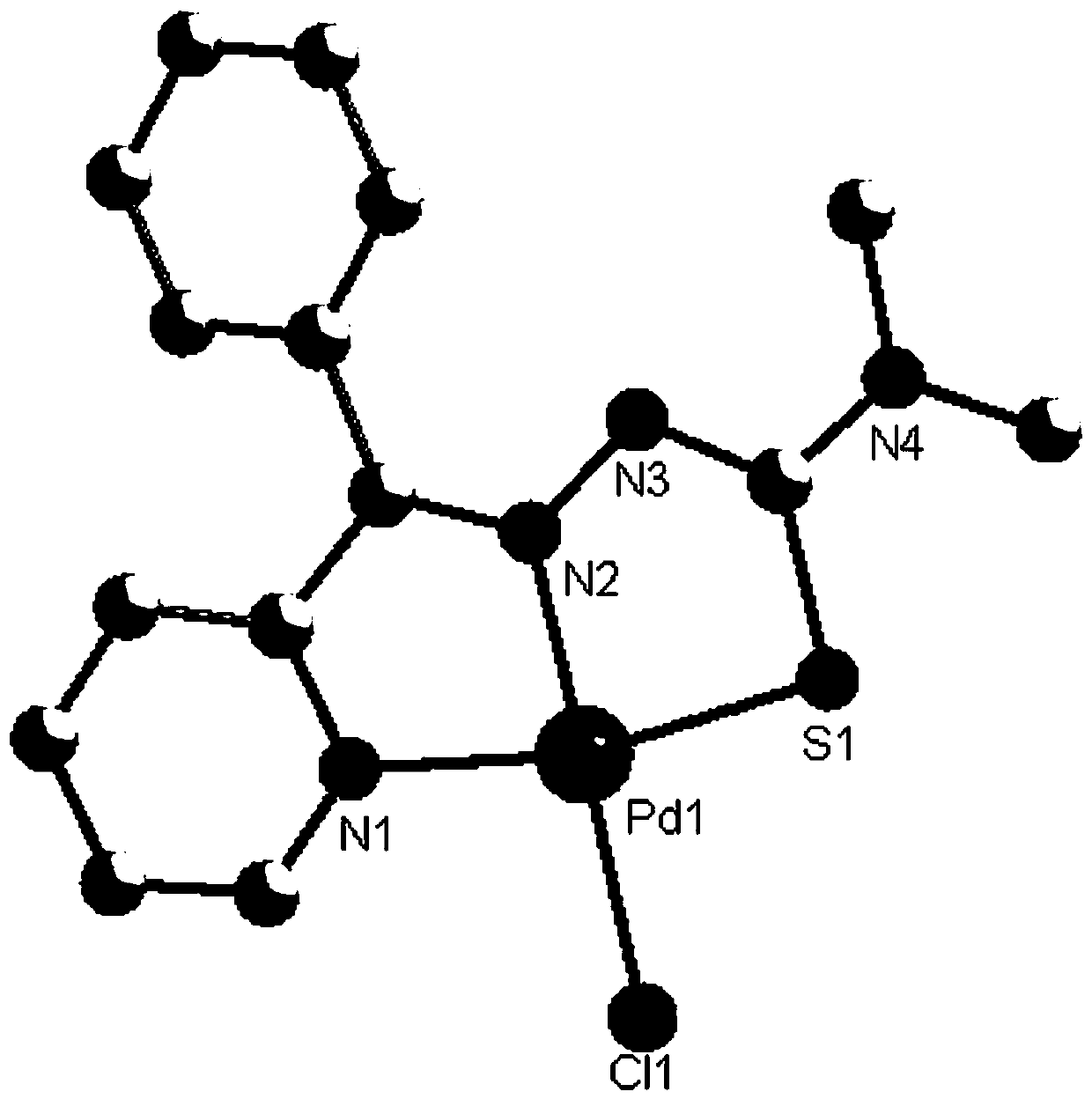
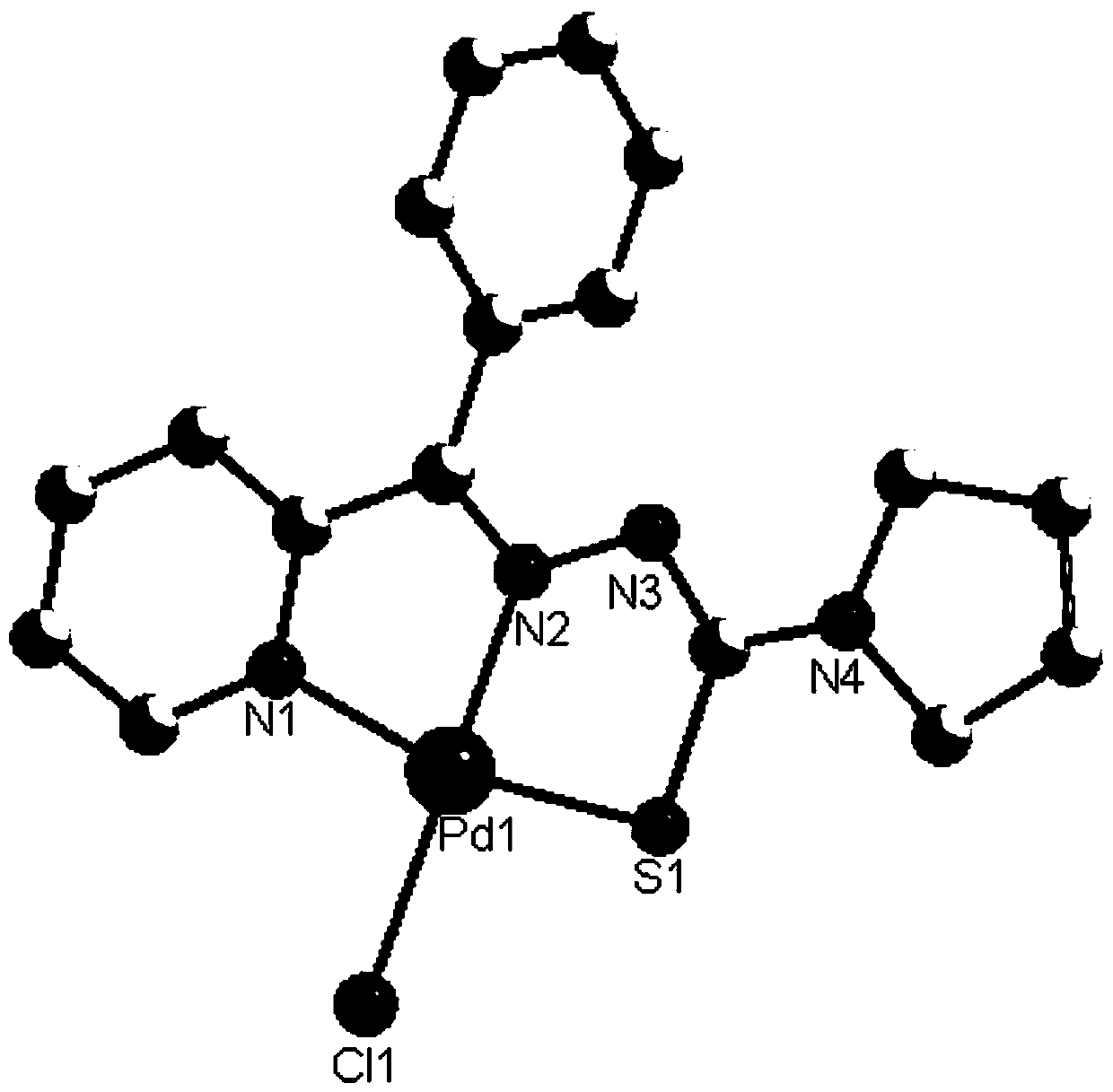
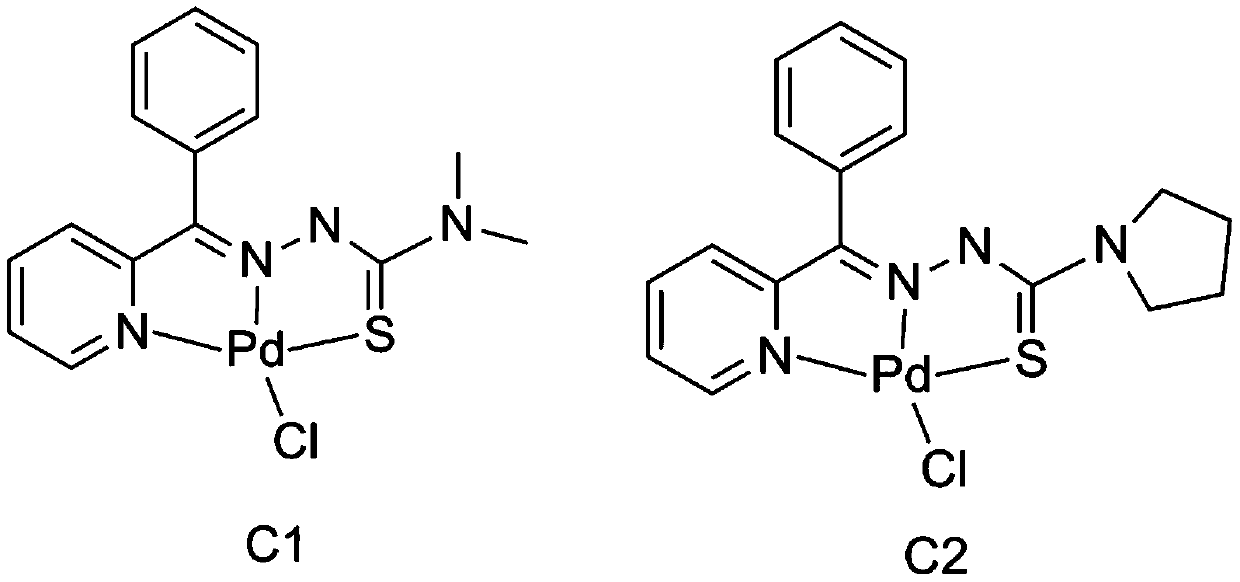
Palladium complexes of 2-benzoylpyridine thiosemicarbazone and synthesis method thereof
A technology of benzoylpyridine thiosemicarbazide and benzoylpyridine, which is applied in the field of palladium complexes with 2-benzoylpyridine thiosemicarbazide and synthesis thereof, can solve problems and accelerate the exploration and development of alternative therapy And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of palladium complex C1 comprises the following steps:
[0026] (1) Mix 3mmol of 2-benzoylpyridine and 3mmol of 4,4-dimethyl-3-thiosemicarbazide, dissolve in 20ml of methanol after mixing, add 500ul of concentrated sulfuric acid dropwise, and stir under reflux at 65°C After reacting for 6 hours, a light yellow precipitate was obtained; the precipitate was filtered, washed with saturated sodium bicarbonate and water successively, and the eluent was petroleum ether:ethyl acetate=10:1, and after drying, the ligand L1 was obtained. Rate: 82%;
[0027] Elemental analysis, Anal.Calcd(%) for C 15 h 16 N 4 S:C,63.35;H,5,67;N,19.70;S,11.28.Found:C,63.28;H,5.11;N,18.75;S,11.14;
[0028] Infrared spectrum, IR, cm -1 :3463(s,amide),3274(s,NH),3208(m,aromatic hydrogen),1526(s),1495(s),1463(s,aromatic),1287(m,C=N),1079 (s, thioamide), 934 (m, C-H), 757 (m, C=S), 694 (m);
[0029] Mass Spectrum, ESI+m / z:C 15 h 16 N 4 S,284.11[M+H] + ;
[0030] (2) Weigh 0.05m...
Embodiment 2
[0035] The synthesis of palladium complex C2 comprises the following steps:
[0036] (1) Mix 3mmol of 2-benzoylpyridine and 3mmol of 3-pyrrole-3-thiosemicarbazide, dissolve in 20ml of methanol after mixing, add 500uL of concentrated sulfuric acid dropwise, and reflux and stir at 65°C for 6h. A light yellow precipitate was obtained; the precipitate was filtered, washed with saturated sodium bicarbonate and water successively after filtration, separated by silica gel column chromatography, and the eluent was petroleum ether:ethyl acetate=10:1, and the ligand L2 was obtained after drying , Yield: 85%;
[0037] Elemental analysis, Anal.Calcd(%) for C 17 h 18 N 4 S: C, 65.78; H, 5.84; N, 18.05; S, 10.33. Found: C, 65.55; H, 5.59; N, 18.27; S, 10.04;
[0038] Infrared spectrum, IR, cm -1 :3355(s,amide),3249(s,NH),3057(m,aromatic hydrogen),1585(s),1469(s),1438(s,aromatic),1236(m,C=N),1154 (s, thioamide), 895 (m, C-H), 797 (m, C=S), 695 (m);
[0039] Mass Spectrum, ESI+m / z:C 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com