Enzyme activity evaluation method for euphausia superba trypsin at low temperature
A technology of trypsin and Antarctic krill, applied in the direction of microorganism-based methods, peptide/protein components, biochemical equipment and methods, etc., can solve the problems of reduced enzyme activity, general healing effect, increased enzyme usage, etc., to achieve improved Safety, high catalytic activity, and high catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0124] The enzyme activity evaluation method of Antarctic krill trypsin under the low temperature of the present embodiment comprises the following steps:
[0125] Step 1: Antarctic krill trypsin genotype acquisition
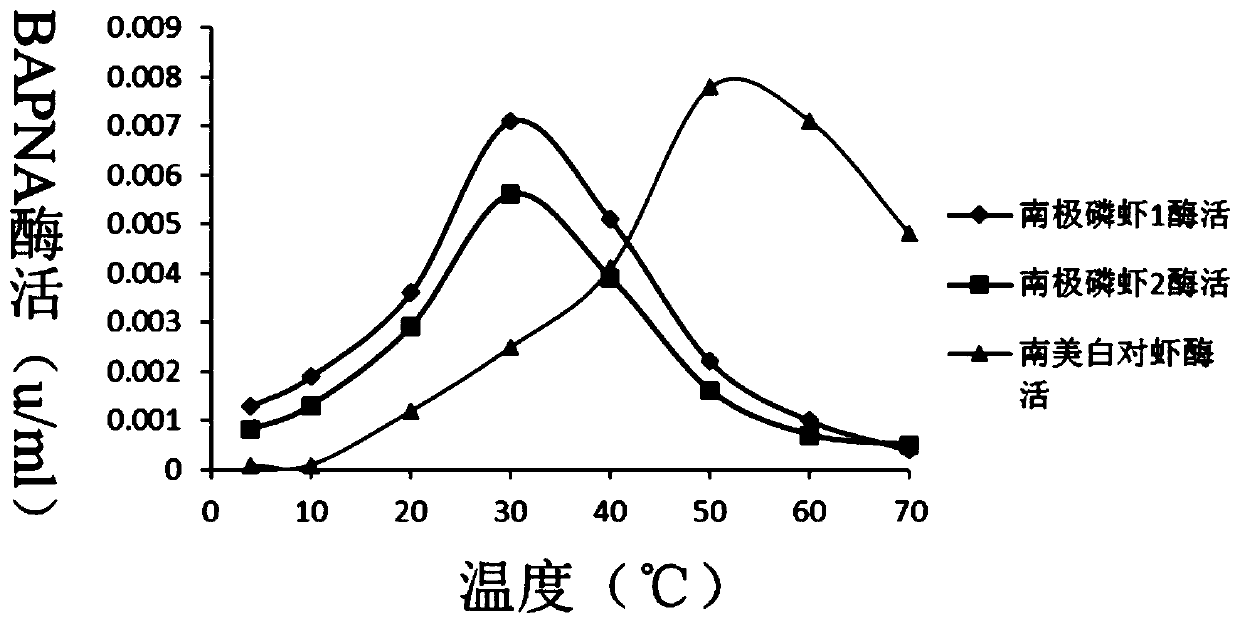
[0126] According to the RACE technology and in combination with the transcriptome data of Antarctic krill of the present invention, two Antarctic krill trypsin subtypes ES-try1 and ES-try2 were obtained, primers with enzyme cutting sites were designed, and these two subtypes were synthesized in vitro Amplified and verified by sequencing. At the same time, using the trypsin from temperate seawater Penaeus vannamei (GenBank: X86369.1) in the NCBI database as a control group, the corresponding trypsin gene coding region was found in NCBI, and then specific primers were designed for PCR in vitro amplification. PCR conditions: 95°C for 5min, 94°C for 30s, 60°C for 30s, 72°C for 1min; 35 cycles, 72°C for 10min, store at 4°C. The PCR product was sequenced and compare...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap