Application of allergen specific IgE antibody idiotypic heterogeneity intensity in kit for allergen detection
An allergen and heterogeneity technology, applied in the field of clinical medicine/experimental diagnostics, can solve problems such as inappropriateness, expensive testing costs, and high technical requirements for operators, and achieve good correlation results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation of embodiment 1 kit
[0042] The kit mainly contains R1 solution, R2 solution and washing buffer, and the specific preparation method is as follows:
[0043] (1) Preparation of R1 solution
[0044] Including the steps of binding different coded fluorescent microspheres (MB, purchased from Merck) coated with SA to different biotin-labeled epitopes (E); the specific method is: 1ml of fluorescent microspheres (concentration: 1mg / mL )+Bio-E (concentration: 1 mg / mL) 100 μL, mix well and incubate with shaking for 1 hour, wash the microspheres, and restore to the original volume with fluorescent microsphere preservation solution. Fluorescent microspheres coated with different epitopes and different codes were mixed in equal proportions, and the concentration of the microspheres was adjusted to 0.01 mg / ml as a working solution to obtain the R1 solution.
[0045] (2) Preparation of R2 solution
[0046] The R2 solution is a phycoerythrin (PE)-labeled mouse anti...
Embodiment 2 Embodiment 1
[0050] The detection method of the use process of kit in embodiment 2 embodiment 1
[0051] The detection steps are as follows:
[0052] (1) Dilute the serum sample with 0.01M pH 7.2 phosphate buffer (containing 5% bovine serum albumin) to dilute the serum sample 1:10, such as 180 μL buffer + 20 μL serum;
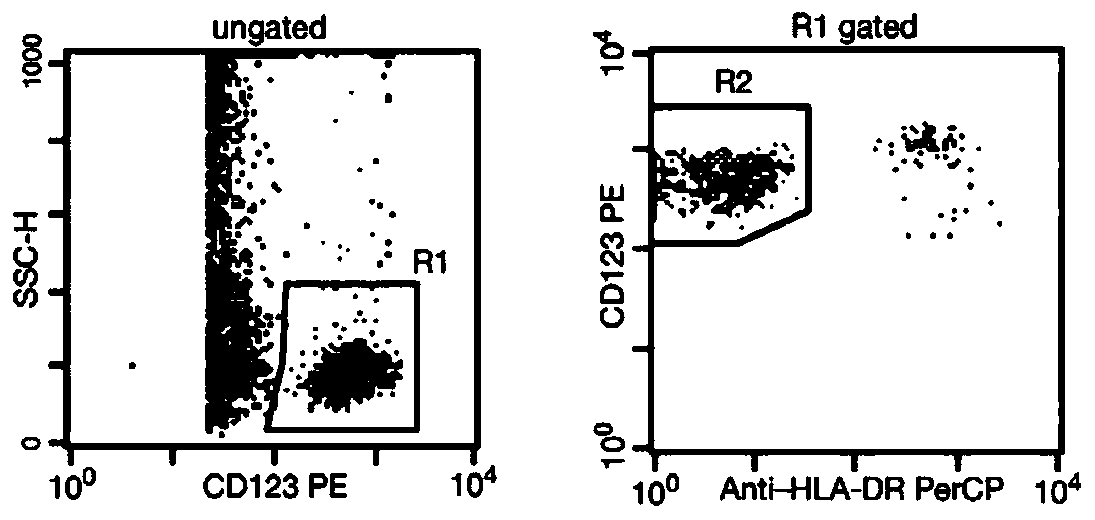
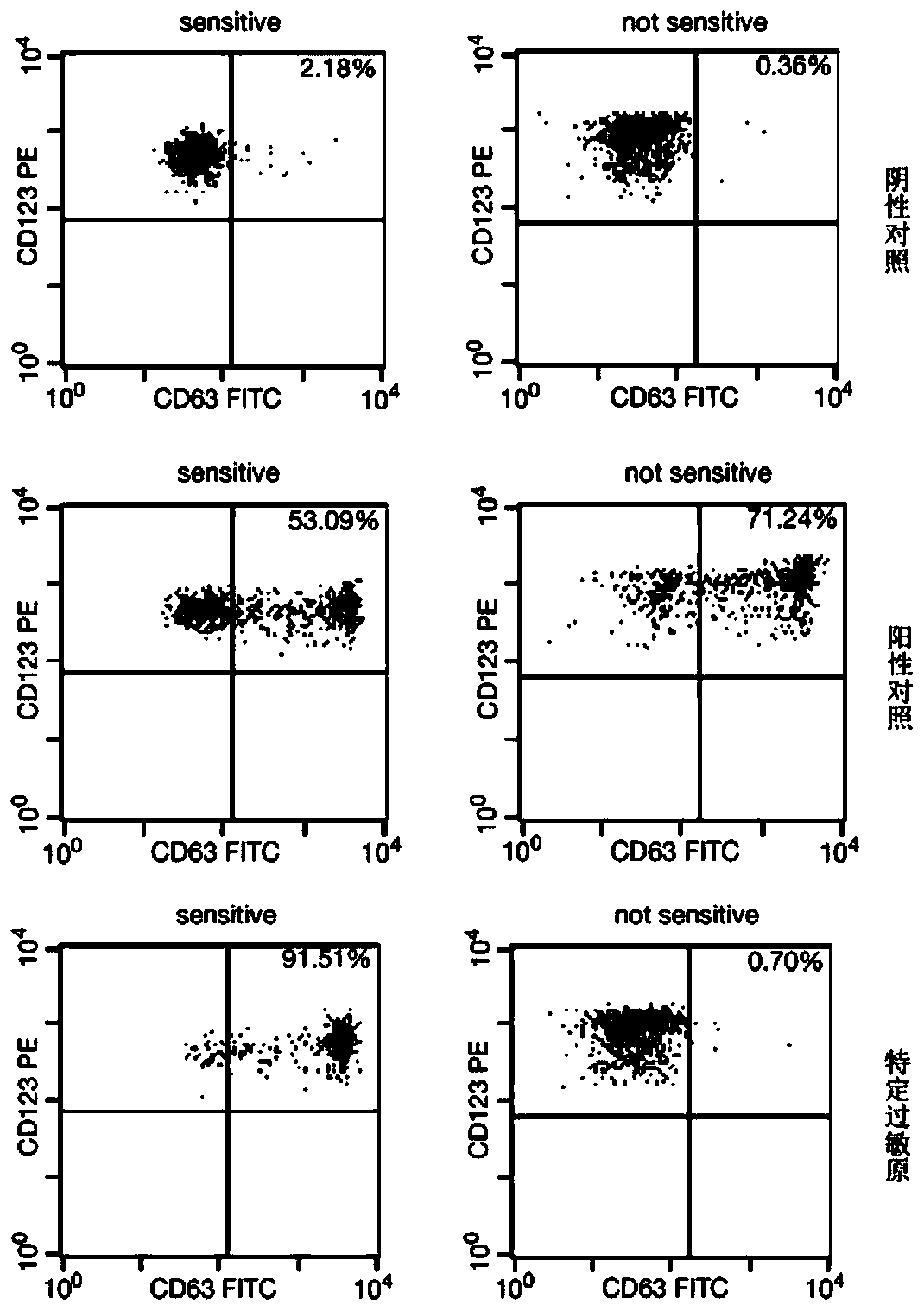
[0053] (2) Add 25 μL of 1:10 diluted serum sample, 100 μL of mixed reagent 1 indirectly coated with different antigenic epitopes and different fluorescent codes into a 96-well plate, and incubate on a plate shaker at 37°C for 1 hour to make The sIgE antibodies with different idiotype epitopes in the serum to be tested combine with the corresponding peptides on the surface of the microspheres one by one to form immune complexes;
[0054] (3) Place the 96-well plate on the magnetic field for 3 minutes, discard the unbound serum protein in the supernatant; after washing three times with washing buffer, discard the supernatant and add 100 μL phosphate buffer (same as above) to...
Embodiment 3 application Embodiment 1
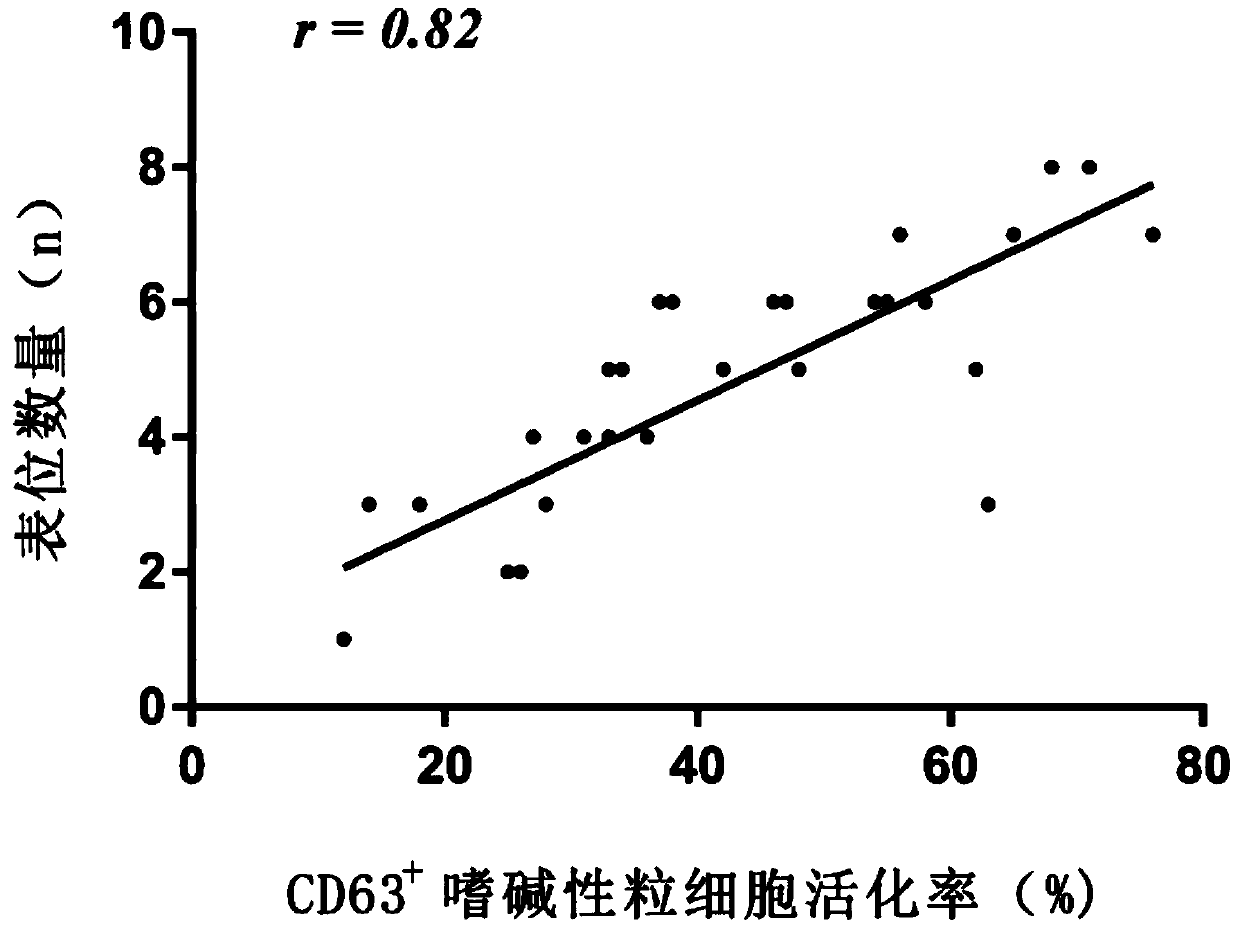
[0061] Example 3 Using the kit in Example 1 and the detection method in Example 2 to detect the heterogeneity intensity of milk allergen β-lactoglobulin sIgE idiotype epitope
[0062] The detection process is as follows:
[0063] (1) The information of the selected epitope is as follows:
[0064] Table: Amino acid sequences of selected β-lactoglobulin epitopes
[0065]
[0066] The amino-terminus was labeled with biotin molecules (Bio-E), and Shanghai Jier Biochemical was commissioned to synthesize these 8 peptides.
[0067] (2) Labeling: purchase 8 different coded fluorescent microspheres (MB 01-08) coated with SA, take 1 mL (concentration: 1 mg / mL) of each and add them to EP tubes, then add 100 μL (concentration: 1 mg / mL) of the above Biotin-labeled antigenic epitopes were incubated at 37°C for 1 hour with shaking. Remove unbound polypeptides by a magnetic field, wash with PBST three times, and restore to the original volume with fluorescent microsphere preservation so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com